| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2009-06-08 14:36:47 UTC |
|---|
| Update Date | 2014-12-24 20:22:52 UTC |
|---|
| Accession Number | T3D0816 |
|---|
| Identification |
|---|
| Common Name | Isobergapten |
|---|
| Class | Small Molecule |
|---|
| Description | Isobergapten is a furocoumarin. Furocoumarins, are phototoxic and photocarcinogenic. They intercalate DNA and photochemically induce mutations. Furocoumarins are botanical phytoalexins found to varying extents in a variety of vegetables and fruits, notably citrus fruits. The levels of furocoumarins present in our diets, while normally well below that causing evident acute phototoxicity, do cause pharmacologically relevant drug interactions. Some are particularly active against cytochrome P450s. For example, in humans, bergamottin and dihydroxybergamottin are responsible for the 'grapefruit juice effect', in which these furanocoumarins affect the metabolism of certain drugs. |
|---|
| Compound Type | - Aromatic Hydrocarbon
- Ester
- Ether
- Food Toxin
- Furocoumarin
- Natural Compound
- Organic Compound
- Plant Toxin
|
|---|
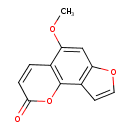
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | 5-Methoxy-2H-furo(2,3-h)-1-benzopyran-2-one | | 5-Methoxy-2H-furo[2,3-H]chromen-2-one | | 5-Methoxyangelicin | | Isobergaptene |
|
|---|
| Chemical Formula | C12H8O4 |
|---|
| Average Molecular Mass | 216.190 g/mol |
|---|
| Monoisotopic Mass | 216.042 g/mol |
|---|
| CAS Registry Number | 482-48-4 |
|---|
| IUPAC Name | 5-methoxy-2H-furo[2,3-h]chromen-2-one |
|---|
| Traditional Name | 5-methoxyfuro[2,3-h]chromen-2-one |
|---|
| SMILES | COC1=CC2=C(C=CO2)C2=C1C=CC(=O)O2 |
|---|
| InChI Identifier | InChI=1S/C12H8O4/c1-14-9-6-10-8(4-5-15-10)12-7(9)2-3-11(13)16-12/h2-6H,1H3 |
|---|
| InChI Key | InChIKey=AJSPSRWWZBBIOR-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as angular furanocoumarins. These are furanocoumarins, with a structure characterized by a furan ring angularly fused to a coumarin. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Coumarins and derivatives |
|---|
| Sub Class | Furanocoumarins |
|---|
| Direct Parent | Angular furanocoumarins |
|---|
| Alternative Parents | |
|---|
| Substituents | - Angular furanocoumarin
- Benzopyran
- 1-benzopyran
- Benzofuran
- Anisole
- Alkyl aryl ether
- Pyranone
- Pyran
- Benzenoid
- Furan
- Heteroaromatic compound
- Lactone
- Oxacycle
- Ether
- Organoheterocyclic compound
- Organic oxygen compound
- Organooxygen compound
- Hydrocarbon derivative
- Organic oxide
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available | | LogP | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00ri-1920000000-05de33e02a65284633bd | 2017-07-27 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0090000000-a7aa4880d1b5f24edee2 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-0090000000-dde9ea19a79896e90ce2 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00rl-0910000000-979b64da744749c2c0c3 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0190000000-a2793a15ec787ff5f199 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-0390000000-ff6e00dbfe68e7c3197f | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00dl-0910000000-20d69a4c35b8a7c99b71 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0090000000-7891ec2b2bad4a52dc5c | 2021-10-21 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-0090000000-7891ec2b2bad4a52dc5c | 2021-10-21 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0300-1910000000-4d4d65f0f318e9f59f8c | 2021-10-21 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0090000000-f3b1b7cd2b3d93168c5f | 2021-10-21 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-0090000000-f3b1b7cd2b3d93168c5f | 2021-10-21 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4r-0900000000-b64840080256c470ec90 | 2021-10-21 | View Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-014i-2590000000-6d348f243bcb82176e41 | 2014-09-20 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Inhibits insect cytochrome P450 (4). The mechanism of action many furocoumarins is based on their ability to form photoadducts with DNA and other cellular components such as RNA, proteins, and several proteins found in the membrane such as phospholipases A2 and C, Ca-dependent and cAMPdependent protein-kinase and epidermal growth factor. Furocoumarins intercalate between base pairs of DNA and after ultraviolet-A irradiation, giving cycloadducts. (4). |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not listed by IARC. IARC has assessed other furocoumarins, classifying 8-methoxypsoralen as carcinogenic to humans (Group 1), 5-methoxypsoralen as possibly carcinogenic to humans (Group 2A), and certain other furocoumarins as not being classifiable as to their carcinogenicity to humans (Group 3). (5) |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | The furocoumarin 8-methoxypsoralen is carcinogenic to humans, and possibly 5-methoxypsoralen as well (5). There is some evidence from mouse studies that other furocoumarins are carcinogenic when combined with exposure to UVA radiation (1). The SKLM regards the additional risk of skin cancer arising from the consumption of typical quantities of furocoumarin-containing foods, which remain significantly below the range of phototoxic doses, as insignificant. However, the consumption of phototoxic quantities cannot be ruled out for certain foods, particularly celery and parsnips, that may lead to significant increases in furocoumarin concentrations, depending on the storage, processing and production conditions. (6) Furocoumarin photochemotherapy is known to induce a number of side-effects including erythema, edema, hyperpigmentation, and premature aging of skin. All photobiological effects of furocoumarins result from their photochemical reactions. Because many dietary or water soluble furocoumarins are strong inhibitors of cytochrome P450s, they will also cause adverse drug reactions when taken with other drugs. |
|---|
| Symptoms | Furocoumarin toxins can cause stomach ache and may also cause a painful skin reaction when contact with the parsnip plant is combined with UV rays from sunlight. (4) |
|---|
| Treatment | Not Available |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| PubChem Compound ID | 68082 |
|---|
| ChEMBL ID | CHEMBL141690 |
|---|
| ChemSpider ID | 61394 |
|---|
| KEGG ID | C18082 |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Isobergapten |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | - Mullen MP, Pathak MA, West JD, Harrist TJ, Dall'Acqua F: Carcinogenic effects of monofunctional and bifunctional furocoumarins. Natl Cancer Inst Monogr. 1984 Dec;66:205-10. [6531030 ]
- Ostertag E, Becker T, Ammon J, Bauer-Aymanns H, Schrenk D: Effects of storage conditions on furocoumarin levels in intact, chopped, or homogenized parsnips. J Agric Food Chem. 2002 Apr 24;50(9):2565-70. [11958623 ]
- Santana L, Uriarte E, Roleira F, Milhazes N, Borges F: Furocoumarins in medicinal chemistry. Synthesis, natural occurrence and biological activity. Curr Med Chem. 2004 Dec;11(24):3239-61. [15579011 ]
- Herboreal Ltd - Manufacturer of rare phytochemicals (2009). [Link]
- International Agency for Research on Cancer (2014). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. [Link]
- DFG Senate Commission on Food Safety (SKLM): Toxicological Assessment of Furocoumarins in Foodstuffs (2006) [Link]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|