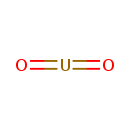| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2009-06-19 21:58:23 UTC |
|---|
| Update Date | 2014-12-24 20:23:15 UTC |
|---|
| Accession Number | T3D1163 |
|---|
| Identification |
|---|
| Common Name | Uranium dioxide |
|---|
| Class | Small Molecule |
|---|
| Description | Uranium dioxide is an oxide of uranium that occurs naturally in the mineral uraninite. It is used for fuel in nuclear reactors. Uranium oxides are also used to colour glass and ceramics. Uranium is a chemical element that has the symbol U and atomic number 92. It is a normal part of rocks, soil, air, and water, and occurs in nature in the form of minerals. (4, 5, 6) |
|---|
| Compound Type | - Industrial/Workplace Toxin
- Inorganic Compound
- Pollutant
- Radioactive
- Synthetic Compound
- Uranium Compound
|
|---|
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | Uranium oxide | | Uranium oxide (UO2) | | Uranium(4) oxide, red | | Uranium(IV) oxide |
|
|---|
| Chemical Formula | O2U |
|---|
| Average Molecular Mass | 270.028 g/mol |
|---|
| Monoisotopic Mass | 270.041 g/mol |
|---|
| CAS Registry Number | 1344-57-6 |
|---|
| IUPAC Name | dioxouranium |
|---|
| Traditional Name | uranium dioxide |
|---|
| SMILES | O=[U]=O |
|---|
| InChI Identifier | InChI=1S/2O.U |
|---|
| InChI Key | InChIKey=FCTBKIHDJGHPPO-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of inorganic compounds known as actinide oxides. These are inorganic compounds containing an oxygen atom of an oxidation state of -2, in which the heaviest atom bonded to the oxygen is an actinide. |
|---|
| Kingdom | Inorganic compounds |
|---|
| Super Class | Mixed metal/non-metal compounds |
|---|
| Class | Actinide organides |
|---|
| Sub Class | Actinide oxides |
|---|
| Direct Parent | Actinide oxides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Actinide oxide
- Inorganic oxide
|
|---|
| Molecular Framework | Not Available |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | Black/brown solid. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | 2865°C (3140°K) | | Boiling Point | Not Available | | Solubility | Not Available | | LogP | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0090000000-e6b8e932dcced60c474e | 2019-02-23 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-0090000000-e6b8e932dcced60c474e | 2019-02-23 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00di-0090000000-e6b8e932dcced60c474e | 2019-02-23 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Oral (5) ; inhalation (5) ; dermal (5) |
|---|
| Mechanism of Toxicity | Uranium is combined with either bicarbonate or a plasma protein in the blood but once in the kidney, it is released and forms complexes with phosphate ligands and proteins in the tubular wall, causing damage. Uranium may also inhibit both sodium transport-dependent and independent ATP utilization and mitochondrial oxidative phosphorylation in the renal proximal tubule. Uranium causes respiratory diseases by damaging alveolar epithelium type II cells in the lungs. Uranium induces c-Jun N-terminal kinase (JNK) and p38 mitogen-activated protein kinase (p38 MAPK) activation, which in turn induces tumor necrosis factor alpha (TNF-alpha) secretion and generates and inflammatory response in the lungs. Studies have shown that the more soluble the uranium salt, the more toxic it is. Ionizing radiation produced by uranium damages the DNA, resulting in gene mutations and chromosomal aberrations. This can both both initiate and promote carcinogenesis, and interfere with reproduction and development. (5, 1) |
|---|
| Metabolism | Uranium is absorbed in low amounts via oral, inhalation, and dermal routes. Uranium in body fluids generally exists as the uranyl ion (UO2)2+ complexed with anions, such as citrate and bicarbonate, or plasma proteins. Uranium preferentially distributes to bone, liver, and kidney. The large majority of uranium that enters the body is not absorbed and is eliminated from the body via the urine and faeces. (4) |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Uranium: Group 1, carcinogenic to humans (7) |
|---|
| Uses/Sources | Uranium dioxide is used for fuel in nuclear reactors. Uranium oxides are also used to colour glass and ceramics. (6) |
|---|
| Minimum Risk Level | Intermediate Inhalation: 0.0004 mg/m3 (Soluble uranium salts) (3)
Chronic Inhalation: 0.0003 mg/m3 (Soluble uranium salts) (3)
Intermediate Oral: 0.002 mg/kg/day (Soluble uranium salts) (3)
Intermediate Inhalation: 0.008 mg/m3 (Insoluble uranium compounds) (3) |
|---|
| Health Effects | Uranium primarily damages the kidney, but may also damage the lungs, central nervous system, and immune system. Uranium's radioactivity is believed to damage the DNA, resulting in carcinogenic effects and reproductive and developmental damage. (4, 5) |
|---|
| Symptoms | Ingestion of uranium may cause vomiting and diarrhea. (4) |
|---|
| Treatment | EYES: irrigate opened eyes for several minutes under running water. INGESTION: do not induce vomiting. Rinse mouth with water (never give anything by mouth to an unconscious person). Seek immediate medical advice. SKIN: should be treated immediately by rinsing the affected parts in cold running water for at least 15 minutes, followed by thorough washing with soap and water. If necessary, the person should shower and change contaminated clothing and shoes, and then must seek medical attention. INHALATION: supply fresh air. If required provide artificial respiration. |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| PubChem Compound ID | 10916 |
|---|
| ChEMBL ID | Not Available |
|---|
| ChemSpider ID | 10454 |
|---|
| KEGG ID | Not Available |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | C012597 |
|---|
| Stitch ID | Uranium dioxide |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | T3D1163.pdf |
|---|
| General References | - Gazin V, Kerdine S, Grillon G, Pallardy M, Raoul H: Uranium induces TNF alpha secretion and MAPK activation in a rat alveolar macrophage cell line. Toxicol Appl Pharmacol. 2004 Jan 1;194(1):49-59. [14728979 ]
- Vidaud C, Dedieu A, Basset C, Plantevin S, Dany I, Pible O, Quemeneur E: Screening of human serum proteins for uranium binding. Chem Res Toxicol. 2005 Jun;18(6):946-53. [15962929 ]
- ATSDR - Agency for Toxic Substances and Disease Registry (2001). Minimal Risk Levels (MRLs) for Hazardous Substances. U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). [Link]
- Wikipedia. Uranium. Last Updated 28 May 2009. [Link]
- ATSDR - Agency for Toxic Substances and Disease Registry (1999). Toxicological profile for uranium. U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). [Link]
- Wikipedia. Uranium dioxide. Last Updated 20 May 2009. [Link]
- International Agency for Research on Cancer (2014). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. [Link]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|