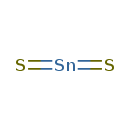| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2009-06-19 21:58:32 UTC |
|---|
| Update Date | 2014-12-24 20:23:33 UTC |
|---|
| Accession Number | T3D1279 |
|---|
| Identification |
|---|
| Common Name | Tin(IV) sulfide |
|---|
| Class | Small Molecule |
|---|
| Description | Tin(IV) sulfide is a sulfide of tin that occurs naturally as the rare mineral berndtite. It is used in decorative coating, where it is known as mosaic gold. Tin is a chemical element with the symbol Sn and atomic number 50. It is a natural component of the earth's crust and is obtained chiefly from the mineral cassiterite, where it occurs as tin dioxide. (2, 4, 5) |
|---|
| Compound Type | - Industrial/Workplace Toxin
- Inorganic Compound
- Natural Compound
- Tin Compound
|
|---|
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | Stannic sulfide | | Tin disulphide | | Tin(IV) sulphide |
|
|---|
| Chemical Formula | S2Sn |
|---|
| Average Molecular Mass | 182.840 g/mol |
|---|
| Monoisotopic Mass | 183.846 g/mol |
|---|
| CAS Registry Number | 1315-01-1 |
|---|
| IUPAC Name | stannanedithione |
|---|
| Traditional Name | stannanedithione |
|---|
| SMILES | S=[Sn]=S |
|---|
| InChI Identifier | InChI=1S/2S.Sn |
|---|
| InChI Key | InChIKey=ALRFTTOJSPMYSY-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of inorganic compounds known as post-transition metal sulfides. These are inorganic compounds containing a sulfur atom of an oxidation state of -2, in which the heaviest atom bonded to the oxygen is a post-transition metal. |
|---|
| Kingdom | Inorganic compounds |
|---|
| Super Class | Mixed metal/non-metal compounds |
|---|
| Class | Post-transition metal organides |
|---|
| Sub Class | Post-transition metal sulfides |
|---|
| Direct Parent | Post-transition metal sulfides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Post-transition metal sulfide
- Inorganic tin salt
- Inorganic sulfide
- Inorganic salt
|
|---|
| Molecular Framework | Not Available |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | <680°C | | Boiling Point | Not Available | | Solubility | Not Available | | LogP | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0900000000-d046589bf47f641333d5 | 2019-02-22 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001i-0900000000-d046589bf47f641333d5 | 2019-02-22 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-001i-0900000000-d046589bf47f641333d5 | 2019-02-22 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Oral (3) ; inhalation (3) ; dermal (3) |
|---|
| Mechanism of Toxicity | Inorganic and organic tin compounds are weak inhibitors of alcohol dehydrogenase. (1) |
|---|
| Metabolism | Though tin metal is very poorly absorbed, tin compounds may be absorbed via oral, inhalation, or dermal routes, with organotin compounds being much more readily absorbed than inorganic tin compounds. Tin may enter the bloodstream and bind to hemoglobin, where it is distributed and accumulates mainly in the kidney, liver, lung, and bone. Tin and its metabolites are excreted mainly in the urine and feces. (3) |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | Tin(IV) sulfide is used in decorative coating, where it is known as mosaic gold. (5)
|
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Ingestion of large amounts of inorganic tin compounds can cause stomachache, anemia, and liver and kidney problems. (2, 3) |
|---|
| Symptoms | Inorganic or organic tin compounds placed on the skin or in the eyes can produce skin and eye irritation. (3) |
|---|
| Treatment | EYES: irrigate opened eyes for several minutes under running water. INGESTION: do not induce vomiting. Rinse mouth with water (never give anything by mouth to an unconscious person). Seek immediate medical advice. SKIN: should be treated immediately by rinsing the affected parts in cold running water for at least 15 minutes, followed by thorough washing with soap and water. If necessary, the person should shower and change contaminated clothing and shoes, and then must seek medical attention. INHALATION: supply fresh air. If required provide artificial respiration. |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| PubChem Compound ID | 25113570 |
|---|
| ChEMBL ID | Not Available |
|---|
| ChemSpider ID | Not Available |
|---|
| KEGG ID | Not Available |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | 50886 |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Tin(IV) sulfide |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | T3D1279.pdf |
|---|
| General References | - Bychkov PV, Shekhovtsova TN, Milaeva ER: Inhibition of horse liver alcohol dehydrogenase by methyltin compounds. Bioinorg Chem Appl. 2005:191-9. doi: 10.1155/BCA.2005.191. [18365099 ]
- Wikipedia. Tributyltin. Last Updated 31 May 2009. [Link]
- ATSDR - Agency for Toxic Substances and Disease Registry (2005). Toxicological profile for tin. U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). [Link]
- Wikipedia. Tin. Last Updated 28 May 2009. [Link]
- Wikipedia. Tin(IV) sulfide. Last Updated 22 January 2009. [Link]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|