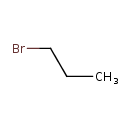| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2009-06-22 16:08:38 UTC |
|---|
| Update Date | 2014-12-24 20:24:39 UTC |
|---|
| Accession Number | T3D1802 |
|---|
| Identification |
|---|
| Common Name | 1-Bromopropane |
|---|
| Class | Small Molecule |
|---|
| Description | 1-bromopropane is an organobromide compound. It is a widely used organic solvent used for the cleaning of metal surfaces, removal of soldering residues from electronic circuit boards, and in the hole transport layer (HTL) of multi-layered OLEDs. It is also a solvent for adhesives and has been deployed as a replacement for perchloroethylene as a dry cleaning solvent. Its use as a solvent in aerosol glues used to glue foam cushions has been especially problematic. 1-bromopropane’s increasing use in the 21st century resulted from need for a substitute for chlorofluorocarbons and perchloroethylene (tetrachloroethylene). In 2013, a peer-review panel convened by the National Toxicology Program unanimously recommended that 1-bromopropane should be classified as a reasonably anticipated human carcinogen. |
|---|
| Compound Type | - Bromide Compound
- Industrial/Workplace Toxin
- Organic Compound
- Organobromide
- Synthetic Compound
|
|---|
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | 1-BROMO-PROPANE | | Bromo propane | | Bromopropane | | n-C3H7Br | | N-propyl bromide | | Propyl bromide |
|
|---|
| Chemical Formula | C3H7Br |
|---|
| Average Molecular Mass | 122.992 g/mol |
|---|
| Monoisotopic Mass | 121.973 g/mol |
|---|
| CAS Registry Number | 106-94-5 |
|---|
| IUPAC Name | 1-bromopropane |
|---|
| Traditional Name | 1-bromopropane |
|---|
| SMILES | CCCBr |
|---|
| InChI Identifier | InChI=1S/C3H7Br/c1-2-3-4/h2-3H2,1H3 |
|---|
| InChI Key | InChIKey=CYNYIHKIEHGYOZ-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as organobromides. Organobromides are compounds containing a chemical bond between a carbon atom and a bromine atom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organohalogen compounds |
|---|
| Class | Organobromides |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Organobromides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Hydrocarbon derivative
- Organobromide
- Alkyl halide
- Alkyl bromide
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | |
|---|
| Biological Roles | |
|---|
| Chemical Roles | |
|---|
| Physical Properties |
|---|
| State | Liquid |
|---|
| Appearance | Colorless liquid. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | -110°C | | Boiling Point | Not Available | | Solubility | 2.45 mg/mL at 20°C [YALKOWSKY,SH & HE,Y (2003)] | | LogP | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0900000000-93ae8c951826e949031d | 2016-06-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-0900000000-ab167994627595e93149 | 2016-06-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9300000000-7b7d969d767e9487c0f5 | 2016-06-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-1900000000-e80db58d1fdc9666b6c2 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-3900000000-ac0f96881b04617b6332 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9100000000-303d9b4d82c8afc6ed07 | 2016-08-03 | View Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-0006-9000000000-01436ff6705673c9ce0e | 2014-09-20 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 90 MHz, CDCl3, experimental) | Not Available | 2014-09-20 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 15.09 MHz, CDCl3, experimental) | Not Available | 2014-09-23 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Oral (9) ; inhalation (9) ; dermal (9) |
|---|
| Mechanism of Toxicity | Organobromide compounds, especially alkylbromides are strong alkylating agents. Consequently they can randomly modify the surfaces of proteins and lipids, leading to the disruption of enzyme, transporter or membrane functions. Alkylation of DNA by alkylbromides may also lead to mutations. 1-bromopropane reacts quickly with hepatic glutathione (GSH) leading to its rapid depletion and subsequent liver damage. Long-term exposure to 1-bromopropane decreases neurogenesis in the dentate gyrus which may contribute to the neurotoxicity. Downregulation of brain derived neurotrophic factor and glucocoritoid receptor mRNA expression and low hippocampal norepinephrine levels might contribute, at least in part, to the reduced neurogenesis. |
|---|
| Metabolism | 1-bromopropane is metabolized rapidly in the liver. Three mercapturic acids are produced: N-acetyl-S-propyl cysteine, N-acetyl-S-propyl cysteine-S-oxide and N-acetyl-S-(2-hydroxypropyl)cysteine. 1-bromopropoane also reacts rapidly with glutathione and can form glutathione conjugates.
|
|---|
| Toxicity Values | LD50: 2.5 g/kg (Intraperitoneal, Mouse) (5)
LC50: 7000 ppm over 4 hours (Inhalation, Rat) (6) |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not listed by IARC. |
|---|
| Uses/Sources | Cleaning solvent, drycleaning solvent, aerosol glue solvent. 1-bromopropane may be found in products used in vapor and immersion degreasing operations for cleaning metal, plastics, electronic and optical components; in adhesive spray applications; and in dry cleaning operations. 1-bromopropane may also be a component of some aerosol spray products. Exposures to 1-bromopropane in occupational settings occur via two primary routes: (1) inhalation of 1-bromopropane vapors and mists; and (2) skin contact with 1-bromopropane.
|
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Skin irritation if contacts skin, redness and itching of skin and eyes. Acute exposure may lead to coughing, shortness of breath, headache, nausea, vomiting. Chronic exposure can lead to damage of the blood, liver and CNS. Neurotoxicity can arise due to chronic exposure through inhalation or skin. Human cases of 1-bromopropane (1-BP) toxicity exhibit ataxic gait and cognitive dysfunction, whereas rat studies showed pyknotic shrinkage in cerebellar Purkinje cells and electrophysiological changes in the hippocampus. Inhalation exposure to 1-bromopropane causes skin tumors in male rats, large intestine tumors in female and male rats, and lung tumors in female mice. 1-bromopropane, either directly or via reactive metabolites, can cause molecular alterations that typically are associated with carcinogenesis, including genotoxicity, oxidative stress, and glutathione depletion. Hepatotoxicity and reproductive toxicity have been noted in rodent studies.
|
|---|
| Symptoms | Reported symptoms to overexposure include confusion, dysarthria, dizziness, paresthesias, and ataxia; unusual fatigue and headaches, development of arthralgias, visual disturbances (difficulty focusing), paresthesias, and muscular twitching. Symptoms may persist over one year after termination of exposure. Vapors may cause dizziness and suffocation. Inhalation of high concentrations may affect behavior/central nervous system (CNS depression) characterized by nausea, headache, dizziness, somnolence, unconsciousness and coma. It may also cause liver and kidney damage, lung injury, weight loss/ anorexia, bone marrow changes, and blood abnormalities.
|
|---|
| Treatment | EYES: irrigate opened eyes for several minutes under running water.
INGESTION: do not induce vomiting. Rinse mouth with water (never give anything by mouth to an unconscious person). Seek immediate medical advice.
SKIN: should be treated immediately by rinsing the affected parts in cold running water for at least 15 minutes, followed by thorough washing with soap and water. If necessary, the person should shower and change contaminated clothing and shoes, and then must seek medical attention.
INHALATION: supply fresh air. If required provide artificial respiration. |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| PubChem Compound ID | 7840 |
|---|
| ChEMBL ID | CHEMBL1230095 |
|---|
| ChemSpider ID | 7552 |
|---|
| KEGG ID | Not Available |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | 47105 |
|---|
| BioCyc ID | BETA-AMINOPROPIONITRILE |
|---|
| CTD ID | C118559 |
|---|
| Stitch ID | n-Propyl bromide |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | 1811 |
|---|
| Wikipedia Link | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | T3D1802.pdf |
|---|
| General References | - Samukawa M, Ichihara G, Oka N, Kusunoki S: A case of severe neurotoxicity associated with exposure to 1-bromopropane, an alternative to ozone-depleting or global-warming solvents. Arch Intern Med. 2012 Sep 10;172(16):1257-60. doi: 10.1001/archinternmed.2012.3987. [22893012 ]
- Zhang L, Nagai T, Yamada K, Ibi D, Ichihara S, Subramanian K, Huang Z, Mohideen SS, Naito H, Ichihara G: Effects of sub-acute and sub-chronic inhalation of 1-bromopropane on neurogenesis in adult rats. Toxicology. 2013 Feb 8;304:76-82. doi: 10.1016/j.tox.2012.12.009. Epub 2012 Dec 21. [23266320 ]
- Liu F, Ichihara S, Valentine WM, Itoh K, Yamamoto M, Sheik Mohideen S, Kitoh J, Ichihara G: Increased susceptibility of Nrf2-null mice to 1-bromopropane-induced hepatotoxicity. Toxicol Sci. 2010 Jun;115(2):596-606. doi: 10.1093/toxsci/kfq075. Epub 2010 Mar 8. [20211940 ]
- Lee SK, Jeon TW, Kim YB, Lee ES, Jeong HG, Jeong TC: Role of glutathione conjugation in the hepatotoxicity and immunotoxicity induced by 1-bromopropane in female BALB/c mice. J Appl Toxicol. 2007 Jul-Aug;27(4):358-67. [17265426 ]
- Bingham, E, Cohrssen, B, and Powell, CH (2001). Patty's Toxicology Volumes 1-9. 5th ed. New York, N.Y: John Wiley & Sons.
- American Conference of Governmental Industrial Hygienists (2008). TLVs and BEIs. Threshold Limit Values for Chemical Substances and Physical Agents and Biological Exposure Indices. Cincinnati, OH: American Conference of Governmental Industrial Hygienists.
- Golomb, BA (1999). A Review of the Scientific Literature As It Pertains to Gulf War Illnesses. Volume 2: Pyridostigmine Bromide. Washington, DC: RAND.
- Wikipedia. n-Propyl bromide. Last Updated 5 June 2009. bromide> [Link]
- International Programme on Chemical Safety (IPCS) INCHEM (1992). Poison Information Monograph for Bromine. [Link]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | | Gene | Gene Symbol | Gene ID | Interaction | Chromosome | Details |
|---|
|
|---|
| Down-Regulated Genes | | Gene | Gene Symbol | Gene ID | Interaction | Chromosome | Details |
|---|
|
|---|