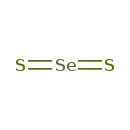| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2009-06-22 16:08:39 UTC |
|---|
| Update Date | 2014-12-24 20:24:41 UTC |
|---|
| Accession Number | T3D1821 |
|---|
| Identification |
|---|
| Common Name | Selenium disulfide |
|---|
| Class | Small Molecule |
|---|
| Description | Selenium disulfide is a sulfide of selenium. It is used an antifungal agent in shampoos for the treatment of dandruff and seborrheic dermatitis. Selenium is a nonmetal element with the atomic number 34 and the chemical symbol Se. Selenium rarely occurs in its elemental state in nature and is usually found in sulfide ores such as pyrite, partially replacing the sulfur in the ore matrix. It may also be found in silver, copper, lead, and nickel minerals. Though selenium salts are toxic in large amounts, trace amounts of the element are necessary for cellular function in most animals, forming the active center of the enzymes glutathione peroxidase, thioredoxin reductase, and three known deiodinase enzymes. (5, 6) |
|---|
| Compound Type | - Household Toxin
- Inorganic Compound
- Non-Metal
- Pollutant
- Selenium Compound
- Synthetic Compound
|
|---|
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | Caswell No. 732A | | Exsel | | Lenium | | Sebusan | | Sel-o-rinse | | Seleen | | Selen(IV) sulfid | | Selenex | | Selenium disulphide | | Selenium sulfide | | Selenium sulfide (SeS2) | | Selenium sulfide SeS2 | | Selenium(IV) disulfide | | Selenix | | Selsun | | Selsun blue | | Selukos | | Sul-blue | | Sulfur selenide | | Zeran |
|
|---|
| Chemical Formula | S2Se |
|---|
| Average Molecular Mass | 143.090 g/mol |
|---|
| Monoisotopic Mass | 143.861 g/mol |
|---|
| CAS Registry Number | 7488-56-4 |
|---|
| IUPAC Name | thione |
|---|
| Traditional Name | selenium disulfide |
|---|
| SMILES | S=[Se]=S |
|---|
| InChI Identifier | InChI=1S/S2Se/c1-3-2 |
|---|
| InChI Key | InChIKey=JNMWHTHYDQTDQZ-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of inorganic compounds known as other non-metal sulfides. These are inorganic compounds containing a sulfur atom of an oxidation state of -2, in which the heaviest atom bonded to the oxygen belongs to the class of other non-metals. |
|---|
| Kingdom | Inorganic compounds |
|---|
| Super Class | Homogeneous non-metal compounds |
|---|
| Class | Other non-metal organides |
|---|
| Sub Class | Other non-metal sulfides |
|---|
| Direct Parent | Other non-metal sulfides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Other non-metal sulfide
- Inorganic sulfide
|
|---|
| Molecular Framework | Not Available |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | 113°C | | Boiling Point | Not Available | | Solubility | Not Available | | LogP | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0900000000-a474aaae5fc0e1be5010 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0006-0900000000-a474aaae5fc0e1be5010 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-0900000000-a474aaae5fc0e1be5010 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0900000000-ba2b59b4dafcf4d22982 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-0900000000-ba2b59b4dafcf4d22982 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-0900000000-ba2b59b4dafcf4d22982 | 2016-08-03 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Oral (4) ; inhalation (4) ; dermal (4) |
|---|
| Mechanism of Toxicity | Selenium readily substitutes for sulfur in biomolecules and in many biochemical reactions, especially when the concentration of selenium is high and the concentration of sulfur is low. Inactivation of the sulfhydryl enzymes necessary for oxidative reactions in cellular respiration, through effects on mitochondrial and microsomal electron transport, might contribute to acute selenium toxicity. Selenomethionine (a common organic selenium compound) also appears to randomly substitute for methionine in protein synthesis. This substitution may affect the structure and functionability of the protein, for example, by altering disulfide bridges. Inorganic forms of selenium appear to react with tissue thiols by redox catalysis, resulting in formation of reactive oxygen species and causing damage by oxidative stress. (4) |
|---|
| Metabolism | Selenium may be absorbed through inhalation and ingestion, while some selenium compounds may also be absorbed dermally. Once in the body, selenium is distributed mainly to the liver and kidney. Selenium is an essential micronutrient and is a component of glutathione peroxidase, iodothyronine 5'-deiodinases, and thioredoxin reductase. Organic selenium is first metabolized into inorganic selenium. Inorganic selenium is reduced stepwise to the intermediate hydrogen selenide, which is either incorporated into selenoproteins after being transformed to selenophosphate and selenocysteinyl tRNA or excreted into the urine after being transformed into methylated metabolites of selenide. Elemental selenium is also methylated before excretion. Selenium is primarily eliminated in the urine and feces, but certain selenium compounds may also be exhaled. (4) |
|---|
| Toxicity Values | LD50: 138 mg/kg (Oral, Rat) (1) |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | 3, not classifiable as to its carcinogenicity to humans. (3) |
|---|
| Uses/Sources | Selenium disulfide is used an antifungal agent in shampoos for the treatment of dandruff and seborrheic dermatitis. (6) |
|---|
| Minimum Risk Level | Chronic Oral: 0.005 mg/kg/day (2) |
|---|
| Health Effects | Chronic oral exposure to high concentrations of selenium compounds can produce a disease called selenosis. The major signs of selenosis are hair loss, nail brittleness, and neurological abnormalities (such as numbness and other odd sensations in the extremities). Animal studies have shown that selenium may also affect sperm production and the female reproductive cycle. (4) |
|---|
| Symptoms | Short-term oral exposure to high concentrations of selenium may cause nausea, vomiting, and diarrhea. Brief exposures to high levels of elemental selenium or selenium dioxide in air can result in respiratory tract irritation, bronchitis, difficulty breathing, and stomach pains. Longer-term exposure to either of these air-borne forms can cause respiratory irritation, bronchial spasms, and coughing. (4) |
|---|
| Treatment | Not Available |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| PubChem Compound ID | 24087 |
|---|
| ChEMBL ID | CHEMBL1200680 |
|---|
| ChemSpider ID | 22519 |
|---|
| KEGG ID | Not Available |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | C025698 |
|---|
| Stitch ID | Selenium disulfide |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | - Lewis RJ (1996). Sax's Dangerous Properties of Industrial Materials. 9th ed. Volumes 1-3. New York, NY: Van Nostrand Reinhold.
- ATSDR - Agency for Toxic Substances and Disease Registry (2001). Minimal Risk Levels (MRLs) for Hazardous Substances. U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). [Link]
- International Agency for Research on Cancer (2014). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. [Link]
- ATSDR - Agency for Toxic Substances and Disease Registry (2003). Toxicological profile for selenium. U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). [Link]
- Wikipedia. Selenium. Last Updated 7 June 2009. [Link]
- Wikipedia. Selenium disulfide. Last Updated 12 June 2009. [Link]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|