| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2009-07-05 03:13:33 UTC |
|---|
| Update Date | 2014-12-24 20:25:42 UTC |
|---|
| Accession Number | T3D2563 |
|---|
| Identification |
|---|
| Common Name | Carbon dioxide |
|---|
| Class | Small Molecule |
|---|
| Description | Carbon dioxide is a colorless, odorless gas that can be formed by the body and is necessary for the respiration cycle of plants and animals. Carbon dioxide is produced during respiration by all animals, fungi and microorganisms that depend on living and decaying plants for food, either directly or indirectly. It is, therefore, a major component of the carbon cycle. Additionally, carbon dioxide is used by plants during photosynthesis to make sugars which may either be consumed again in respiration or used as the raw material to produce polysaccharides such as starch and cellulose, proteins and the wide variety of other organic compounds required for plant growth and development. When inhaled at concentrations much higher than usual atmospheric levels, it can produce a sour taste in the mouth and a stinging sensation in the nose and throat. These effects result from the gas dissolving in the mucous membranes and saliva, forming a weak solution of carbonic acid. Carbon dioxide is used by the food industry, the oil industry, and the chemical industry. Carbon dioxide is used to produce carbonated soft drinks and soda water. Traditionally, the carbonation in beer and sparkling wine comes about through natural fermentation, but some manufacturers carbonate these drinks artificially. |
|---|
| Compound Type | - Food Toxin
- Household Toxin
- Industrial/Workplace Toxin
- Metabolite
- Natural Compound
- Organic Compound
- Vapour
|
|---|
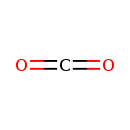
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | Carbon oxide | | Carbon-12 dioxide | | Carbonic acid anhydride | | Carbonic acid gas | | Carbonic anhydride |
|
|---|
| Chemical Formula | CO2 |
|---|
| Average Molecular Mass | 44.010 g/mol |
|---|
| Monoisotopic Mass | 43.990 g/mol |
|---|
| CAS Registry Number | 124-38-9 |
|---|
| IUPAC Name | methanedione |
|---|
| Traditional Name | carbon dioxide |
|---|
| SMILES | O=C=O |
|---|
| InChI Identifier | InChI=1S/CO2/c2-1-3 |
|---|
| InChI Key | InChIKey=CURLTUGMZLYLDI-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of inorganic compounds known as other non-metal oxides. These are inorganic compounds containing an oxygen atom of an oxidation state of -2, in which the heaviest atom bonded to the oxygen belongs to the class of 'other non-metals'. |
|---|
| Kingdom | Inorganic compounds |
|---|
| Super Class | Homogeneous non-metal compounds |
|---|
| Class | Other non-metal organides |
|---|
| Sub Class | Other non-metal oxides |
|---|
| Direct Parent | Other non-metal oxides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Other non-metal oxide
- Inorganic oxide
|
|---|
| Molecular Framework | Not Available |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Endogenous |
|---|
| Cellular Locations | - Cytoplasm
- Endoplasmic reticulum
- Extracellular
- Golgi apparatus
- Mitochondria
- Nucleus
- Peroxisome
|
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | |
|---|
| Pathways | |
|---|
| Applications | |
|---|
| Biological Roles | |
|---|
| Chemical Roles | |
|---|
| Physical Properties |
|---|
| State | Liquid |
|---|
| Appearance | Colorless, odorless gas (7). |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | -56.5°C | | Boiling Point | Not Available | | Solubility | 1.48 mg/mL at 25°C | | LogP | 0.83 |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0006-9000000000-ffb540455919d1e43969 | 2017-09-12 | View Spectrum | | GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0006-9000000000-ffb540455919d1e43969 | 2018-05-18 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0006-9000000000-9afc44eaa1fe34c4f458 | 2016-09-22 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-9000000000-eb2207f7400e9144fff7 | 2015-05-26 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0006-9000000000-eb2207f7400e9144fff7 | 2015-05-26 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9000000000-eb2207f7400e9144fff7 | 2015-05-26 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-9000000000-b7e6c1e22f1f90c5a8e0 | 2015-05-27 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-9000000000-b7e6c1e22f1f90c5a8e0 | 2015-05-27 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9000000000-b7e6c1e22f1f90c5a8e0 | 2015-05-27 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-9000000000-a1e091bb1f5fa6e9cbc7 | 2021-09-24 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0006-9000000000-a1e091bb1f5fa6e9cbc7 | 2021-09-24 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9000000000-a1e091bb1f5fa6e9cbc7 | 2021-09-24 | View Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-0006-9000000000-e1cf88df1066f206d01f | 2014-09-20 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Inhalation (7) ; dermal (7) ; eye contact (7). |
|---|
| Mechanism of Toxicity | Carbon dioxide causes widespread activation of the sympathetic nervous system and an increase in the plasma concentrations of epinephrine, norepinephrine, angiotensin, and other vasoactive peptides . The response is mediated by various subcortical centers in the hypothalamus, brainstem reticular formation and medulla. These areas can be excited locally by carbon dioxide, but they also receive afferents from the carotid and aortic chemoreceptors that are sensitive to changes in carbon dioxide in the blood. The results of sympathetic nervous system activation are, in general, opposite to the local effects of carbon dioxide. The sympathetic effects consist of an increase in cardiac contractility and heart rate and vasoconstriction (3). |
|---|
| Metabolism | Carbon dioxide is transported in the blood in diverse forms: dissolved in the plasma, or linked to proteins independently of the PCO2. Carbone dioxide is transported by the hemoglobin back to the lungs, where it is exhaled (2, 10). |
|---|
| Toxicity Values | LC50: 470 000 ppm (Inhalation, Rat) (9) |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity (not listed by IARC). (6) |
|---|
| Uses/Sources | Carbon dioxide is used by the food industry, the oil industry, and the chemical industry. It is used in many consumer products that require pressurized gas. Life jackets often contain canisters of pressured carbon dioxide for quick inflation. Aluminum capsules are also sold as supplies of compressed gas for airguns, paintball markers, for inflating bicycle tires, and for making seltzer (7). |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Carbon dioxide poisoning (Hypercapnia) can induce increased cardiac output, an elevation in arterial blood pressure, and a propensity toward arrhythmias (8). |
|---|
| Symptoms | Flushed skin, full pulse, extrasystoles, muscle twitches, hand flaps, reduced neural activity, headache, and possibly a raised blood pressure. In severe poisoning, symptomatology progresses to disorientation, panic, hyperventilation, convulsions, unconsciousness, and eventually death (8). |
|---|
| Treatment | In case of inhalation, administer 100% humidified supplemental oxygen with assisted ventilation as required. Administer a benzodiazepine IV if seizures occur. Irrigate exposed eyes with copious amounts of room temperature water for at least 15 minutes if exposure occurred through eye exposure. In case of dermal exposure, rewarming and a variety of topical treatments are indicated for frostbite injury. (4)
|
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB01967 |
|---|
| PubChem Compound ID | 280 |
|---|
| ChEMBL ID | CHEMBL1231871 |
|---|
| ChemSpider ID | 274 |
|---|
| KEGG ID | C00011 |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | 141250 , 172460 , 259900 , 266500 |
|---|
| ChEBI ID | 16526 |
|---|
| BioCyc ID | CARBON-MONOXIDE |
|---|
| CTD ID | D002245 |
|---|
| Stitch ID | Carbon dioxide |
|---|
| PDB ID | CO2 |
|---|
| ACToR ID | 3647 |
|---|
| Wikipedia Link | Carbon Dioxide |
|---|
| References |
|---|
| Synthesis Reference | Callahan, Richard A. Process and apparatus for producing liquid carbon dioxide. U.S. (1993), 11 pp. |
|---|
| MSDS | Link |
|---|
| General References | - Ozensoy O, Arslan O, Kockar F: Differential in vitro inhibition effects of some antibiotics on tumor associated carbonic anhydrase isozymes of hCA-IX and hCA-XII. J Enzyme Inhib Med Chem. 2008 Aug;23(4):579-85. doi: 10.1080/14756360701731957 . [18666004 ]
- Balasubramanian M, Moorthy PS, Neelagandan K, Ponnuswamy MN: Preliminary Crystallographic Study of Hemoglobin from Buffalo (Bubalus bubalis): A Low Oxygen Affinity Species. Protein Pept Lett. 2009;16(2):213-5. [19200047 ]
- Sato K, Yoshida K, Takahashi H, Ito K, Kamata M, Higuchi H, Shimizu T, Itoh K, Inoue K, Tezuka T, Suzuki T, Ohkubo T, Sugawara K, Otani K: Association between -1438G/A promoter polymorphism in the 5-HT(2A) receptor gene and fluvoxamine response in Japanese patients with major depressive disorder. Neuropsychobiology. 2002;46(3):136-40. [12422060 ]
- Rumack BH (2009). POISINDEX(R) Information System. Englewood, CO: Micromedex, Inc. CCIS Volume 141, edition expires Aug, 2009.
- Gilman AG, Rall TW, Nies AS and Taylor P (eds) (1990). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press.
- International Agency for Research on Cancer (2014). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. [Link]
- Wikipedia. Carbon dioxide. Last Updated 5 August 2009. [Link]
- Wikipedia. Carbon dioxide poisoning. Last Updated 5 August 2009. [Link]
- Air Gas (2009). Material Safety Data Sheet. Carbon Dioxide / Nitrogen Dioxide / Oxygen / Sulfur Dioxide. [Link]
- Scherperell, Philippe (2009). Carbon Dioxide Metabolism - Capnography. [Link]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|