| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2009-07-15 20:43:44 UTC |
|---|
| Update Date | 2014-12-24 20:25:48 UTC |
|---|
| Accession Number | T3D2667 |
|---|
| Identification |
|---|
| Common Name | Ibandronate |
|---|
| Class | Small Molecule |
|---|
| Description | Ibandronate is a nitrogen-containing bisphosphonate in the same class as alendronate and risedronate. Ibandronate inhibits osteoclast-mediated bone resorption. All of the bisphosphonates prevent the breakdown of bone by bone cells called osteoclasts. In persons who are at high risk for osteoporosis, bisphosphonates not only result in increased amounts of bone and bone strength, they also reduce the risk of hip fractures and other bone fractures. |
|---|
| Compound Type | - Amine
- Antihypocalcemic Agent
- Antiresorptive
- Bisphosphonate
- Bone Density Conservation Agent
- Drug
- Metabolite
- Organic Compound
- Synthetic Compound
|
|---|
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | ADRONiL | | Bondronat | | Boniva | | Bonviva | | Ibandronate sodium monohydrate | | Ibandronic acid | | R484 |
|
|---|
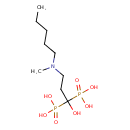
| Chemical Formula | C9H23NO7P2 |
|---|
| Average Molecular Mass | 319.229 g/mol |
|---|
| Monoisotopic Mass | 319.095 g/mol |
|---|
| CAS Registry Number | 114084-78-5 |
|---|
| IUPAC Name | {1-hydroxy-3-[methyl(pentyl)amino]-1-phosphonopropyl}phosphonic acid |
|---|
| Traditional Name | ibandronate |
|---|
| SMILES | CCCCCN(C)CCC(O)(P(O)(O)=O)P(O)(O)=O |
|---|
| InChI Identifier | InChI=1S/C9H23NO7P2/c1-3-4-5-7-10(2)8-6-9(11,18(12,13)14)19(15,16)17/h11H,3-8H2,1-2H3,(H2,12,13,14)(H2,15,16,17) |
|---|
| InChI Key | InChIKey=MPBVHIBUJCELCL-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as bisphosphonates. These are organic compounds containing two phosphonate groups linked together through a carbon atoms. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Organic phosphonic acids and derivatives |
|---|
| Sub Class | Bisphosphonates |
|---|
| Direct Parent | Bisphosphonates |
|---|
| Alternative Parents | |
|---|
| Substituents | - Bisphosphonate
- Organophosphonic acid
- 1,3-aminoalcohol
- Tertiary aliphatic amine
- Tertiary amine
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Organophosphorus compound
- Organooxygen compound
- Organonitrogen compound
- Amine
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | | Name | SMPDB Link | KEGG Link |
|---|
| Ibandronate Pathway | Not Available | Not Available |
|
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Freely soluble | | LogP | -2.1 |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-08gl-9130000000-f1a145132fe5a1d4852e | 2017-09-01 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-0233-9113000000-5576d5d18472e7b11a4a | 2017-10-06 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-1089000000-b2afb60f04d1d7076014 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0080-7790000000-037f20f19b338e3a638b | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01c3-9120000000-6b11acb03cc3d07ff611 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014r-1159000000-86d2ef11326a8e83cda4 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udr-4694000000-cd166d728f1d865e6ce5 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-003r-9000000000-d06bd55271aea4ebdb18 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-1009000000-81303b095a47932563f4 | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-0019000000-df5f634d806e75d58992 | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0597-9100000000-98c63a25e8164e665af3 | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0009000000-2f3bcd85bd99ea1851c6 | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0gdi-5009000000-06df6dec77c09a2d386c | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0fai-9131000000-ba46adab8d9bdbd3c87c | 2021-10-11 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | 2021-09-29 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | 2021-09-29 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | 2021-09-29 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | 2021-09-29 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | 2021-09-29 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | 2021-09-29 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | 2021-09-29 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | 2021-09-29 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | 2021-09-29 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | 2021-09-29 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | 2021-09-29 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | 2021-09-29 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | 2021-09-29 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | 2021-09-29 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | 2021-09-29 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | 2021-09-29 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | 2021-09-29 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | 2021-09-29 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | 2021-09-29 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | 2021-09-29 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Poorly absorbed (mean bioavailability following a 2.5 mg oral dose is about 0.6% compared to intravenous dosing). Absorption is impaired by any kind of food or drink other than plain water. |
|---|
| Mechanism of Toxicity | The action of ibandronate on bone tissue is based partly on its affinity for hydroxyapatite, which is part of the mineral matrix of bone. Nitrogen-containing bisphosphonates (such as pamidronate, alendronate, risedronate, ibandronate and zoledronate) appear to act as analogues of isoprenoid diphosphate lipids, thereby inhibiting farnesyl pyrophosphate (FPP) synthase, an enzyme in the mevalonate pathway. Inhibition of this enzyme in osteoclasts prevents the biosynthesis of isoprenoid lipids (FPP and GGPP) that are essential for the post-translational farnesylation and geranylgeranylation of small GTPase signalling proteins. This activity inhibits osteoclast activity and reduces bone resorption and turnover. In postmenopausal women, it reduces the elevated rate of bone turnover, leading to, on average, a net gain in bone mass. |
|---|
| Metabolism | No evidence of ibandronate being metabolized in humans.
Route of Elimination: Ibandronate is eliminated by renal excretion. Unabsorbed ibandronate is eliminated unchanged in the feces.
Half Life: 10-60 hours |
|---|
| Toxicity Values | LD50 = 811 mg/kg (rat, oral) |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | For the treatment and prevention of osteoporosis in postmenopausal women. |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | LD50 = 811 mg/kg (rat, oral), side effects include bronchitis, pneumonia and urinary tract infections. |
|---|
| Treatment | Not Available |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB00710 |
|---|
| HMDB ID | HMDB14848 |
|---|
| PubChem Compound ID | 60852 |
|---|
| ChEMBL ID | CHEMBL997 |
|---|
| ChemSpider ID | 54839 |
|---|
| KEGG ID | Not Available |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Ibandronic acid |
|---|
| PDB ID | BFQ |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Ibandronate |
|---|
| References |
|---|
| Synthesis Reference | Revital Lifshitz-Liron, Thomas Bayer, Judith Aronhime, Michael Pinchasov, “Solid and crystalline ibandronate sodium and processes for preparation thereof.” U.S. Patent US20070179119, issued August 02, 2007. |
|---|
| MSDS | Link |
|---|
| General References | - Wishart DS, Knox C, Guo AC, Cheng D, Shrivastava S, Tzur D, Gautam B, Hassanali M: DrugBank: a knowledgebase for drugs, drug actions and drug targets. Nucleic Acids Res. 2008 Jan;36(Database issue):D901-6. Epub 2007 Nov 29. [18048412 ]
- Epstein S, Zaidi M: Biological properties and mechanism of action of ibandronate: application to the treatment of osteoporosis. Bone. 2005 Oct;37(4):433-40. [16046205 ]
- Drugs.com [Link]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|