| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2009-07-21 20:26:07 UTC |
|---|
| Update Date | 2014-12-24 20:25:49 UTC |
|---|
| Accession Number | T3D2692 |
|---|
| Identification |
|---|
| Common Name | Calcitriol |
|---|
| Class | Small Molecule |
|---|
| Description | Calcitriol or 1,25-dihydroxycholecalciferol (abbreviated 1,25-(OH)2-D3) is the active form of vitamin D found in the body (vitamin D3). Calcitriol is marketed under various trade names including Rocaltrol (Roche), Calcijex (Abbott) and Decostriol (Mibe, Jesalis). It is produced in the kidneys via 25-hydroxyvitamin D-1 α-hydroxylase by conversion from 25-hydroxycholecalciferol (calcidiol). This is stimulated by a decrease in serum calcium, phosphate (PO43-) and parathyroid hormone (PTH) levels. It regulates calcium levels by increasing the absorption of calcium and phosphate from the gastrointestinal tract, increasing calcium and phosphate reabsorption in the kidneys and inhibiting the release of PTH. Calcitriol is also commonly used as a medication in the treatment of hypocalcemia and osteoporosis. |
|---|
| Compound Type | - Antihypocalcemic Agent
- Antihypoparathyroid Agent
- Antithyroid Agent
- Bone Density Conservation Agent
- Calcium Channel Agonist
- Drug
- Essential Vitamin
- Food Toxin
- Metabolite
- Natural Compound
- Nutraceutical
- Organic Compound
- Vitamin
- Vitamin D
|
|---|
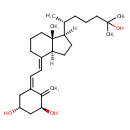
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | (1alpha,3beta,5Z,7e)-9,10-Secocholesta-5,7,10(19)-triene-1,3,25-triol | | (1S,3R,5Z,7e)-9,10-Secocholesta-5,7,10-triene-1,3,25-triol | | (3b,5Z,7E)-9,10-Secocholesta-5,7,10(19)-trienetriol | | (5Z,7e)-(1S,3R)-9,10-Secocholesta-5,7,10(19)-triene-1,3,25-triol | | 1,25 Dihydroxycholecalciferol | | 1,25-DHCC | | 1,25-Dihydroxycholecalciferol | | 1,25-Dihydroxyvitamin D | | 1,25-Dihydroxyvitamin D3 | | 1-alpha,25-Dihydroxyvitamin D3 | | 1-alpha-25-Dihydroxyvitamin D3 | | 1a,25-(OH)2D3 | | 1a,25-Dihydroxycholecalciferol | | 1a,25-Dihydroxyvitamin D3 | | 1alpha,25(OH)2D3 | | 1alpha,25-Dihydroxycholecalciferol | | 1alpha,25-Dihydroxyvitamin D3 | | 1α,25(OH)2D3 | | 1α,25-dihydroxycholecalciferol | | 1α,25-dihydroxyvitamin D3 | | 25-Dihydroxycholecalciferol | | 5-{2-[1-(5-hydroxy-1,5-dimethyl-hexyl)-7a-methyl-octahydro-inden-4-ylidene]-ethylidene}-4-methylene-cyclohexane-1,3-diol | | Calcijex | | Calcitriol Oral Solution | | Calcitriolum | | Decostriol | | Dihydroxyvitamin D3 | | Ro 21-5535 | | Rocaltrol | | Silkis | | Soltriol | | Topitriol | | Toptriol | | Vectical |
|
|---|
| Chemical Formula | C27H44O3 |
|---|
| Average Molecular Mass | 416.637 g/mol |
|---|
| Monoisotopic Mass | 416.329 g/mol |
|---|
| CAS Registry Number | 32222-06-3 |
|---|
| IUPAC Name | (1R,3S,5Z)-5-{2-[(1R,3aS,4E,7aR)-1-[(2R)-6-hydroxy-6-methylheptan-2-yl]-7a-methyl-octahydro-1H-inden-4-ylidene]ethylidene}-4-methylidenecyclohexane-1,3-diol |
|---|
| Traditional Name | calcitriol |
|---|
| SMILES | [H]\C(\C(\[H])=C1/CCC[C@@]2(C)[C@@]1([H])CC[C@]2([H])[C@]([H])(C)CCCC(C)(C)O)=C1/C[C@@]([H])(O)C[C@]([H])(O)C1=C |
|---|
| InChI Identifier | InChI=1S/C27H44O3/c1-18(8-6-14-26(3,4)30)23-12-13-24-20(9-7-15-27(23,24)5)10-11-21-16-22(28)17-25(29)19(21)2/h10-11,18,22-25,28-30H,2,6-9,12-17H2,1,3-5H3/b20-10+,21-11-/t18-,22-,23-,24+,25+,27-/m1/s1 |
|---|
| InChI Key | InChIKey=GMRQFYUYWCNGIN-NKMMMXOESA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as vitamin d and derivatives. Vitamin D and derivatives are compounds containing a secosteroid backbone, usually secoergostane or secocholestane. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Vitamin D and derivatives |
|---|
| Direct Parent | Vitamin D and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Triterpenoid
- Tertiary alcohol
- Cyclic alcohol
- Secondary alcohol
- Organic oxygen compound
- Hydrocarbon derivative
- Organooxygen compound
- Alcohol
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Endogenous |
|---|
| Cellular Locations | - Extracellular
- Membrane
- Mitochondria
|
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | - Adrenal Medulla
- Bladder
- Fibroblasts
- Gonads
- Gut
- Intestine
- Kidney
- Muscle
- Pancreas
- Placenta
- Prostate
- Skeletal Muscle
- Skin
- Spleen
- Stratum Corneum
- Testes
|
|---|
| Pathways | Not Available |
|---|
| Applications | |
|---|
| Biological Roles | |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | 113-114°C | | Boiling Point | Not Available | | Solubility | Insoluble | | LogP | 5 |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0aba-2019200000-f91cfe8e2e00e1a8f6da | 2017-09-01 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-014i-2200139000-8f9b07740968867d2e47 | 2017-10-06 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001j-0009100000-5d85e3fe2656c4f2c798 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001j-0239000000-16579742f7806ac7c580 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0080-3297000000-1ded6f55cc278e5facb8 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0006900000-58dff5fdfef8e2a6c7c9 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00kb-0009300000-93004eb4d3b2dee27fe8 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000t-1119000000-a3201818a6bc9ce6cac4 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001j-0119000000-f58eb6212ebcce40560e | 2021-09-22 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014r-4396100000-7177c9113a57f65c8c66 | 2021-09-22 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01w1-1961000000-2ac1cc3761902eada6c4 | 2021-09-22 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0000900000-67ea14fb7a26b9b2d929 | 2021-09-23 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-0505900000-160a8d14ac12dfdfe86e | 2021-09-23 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-02k9-0839500000-5c13b18b5994b1b65288 | 2021-09-23 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Intravenous, Oral.
Rapidly absorbed from the intestine. |
|---|
| Mechanism of Toxicity | The mechanism of action of calcitriol in the treatment of psoriasis is accounted for by their antiproliferative activity for keratinocytes and their stimulation of epidermal cell differentiation. The anticarcinogenic activity of the active form of Calcitriol appears to be correlated with cellular vitamin D receptor (VDR) levels. Vitamin D receptors belong to the superfamily of steroid-hormone zinc-finger receptors. VDRs selectively bind 1,25-(OH)2-D3 and retinoic acid X receptor (RXR) to form a heterodimeric complex that interacts with specific DNA sequences known as vitamin D-responsive elements. VDRs are ligand-activated transcription factors. The receptors activate or repress the transcription of target genes upon binding their respective ligands. It is thought that the anticarcinogenic effect of Calcitriol is mediated via VDRs in cancer cells. The immunomodulatory activity of calcitriol is thought to be mediated by vitamin D receptors (VDRs) which are expressed constitutively in monocytes but induced upon activation of T and B lymphocytes. 1,25-(OH)2-D3 has also been found to enhance the activity of some vitamin D-receptor positive immune cells and to enhance the sensitivity of certain target cells to various cytokines secreted by immune cells. |
|---|
| Metabolism | The first pathway involves 24-hydroxylase activity in the kidney; this enzyme is also present in many target tissues which possess the vitamin D receptor such as the intestine. The end product of this pathway is a side chain shortened metabolite, calcitroic acid. The second pathway involves the conversion of calcitriol via the stepwise hydroxylation of carbon-26 and carbon-23, and cyclization to yield ultimately 1a,25R(OH)2-26,23S-lactone D3. The lactone appears to be the major metabolite circulating in humans.
Route of Elimination: Enterohepatic recycling and biliary excretion of calcitriol occur. The metabolites of calcitriol are excreted primarily in feces. Cumulative excretion of radioactivity on the sixth day following intravenous administration of radiolabeled calcitriol averaged 16% in urine and 49% in feces.
Half Life: 5-8 hours |
|---|
| Toxicity Values | LD50 (oral, rat) = 620 μg/kg; LD50 (intraperitoneal, rat) > 5 mg/kg. |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | Used to treat vitamin D deficiency or insufficiency, refractory rickets (vitamin D resistant rickets), familial hypophosphatemia and hypoparathyroidism, and in the management of hypocalcemia and renal osteodystrophy in patients with chronic renal failure undergoing dialysis. Also used in conjunction with calcium in the management and prevention of primary or corticosteroid-induced osteoporosis. |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Overdose evident in elevated blood calcium levels causing symptoms of anorexia, nausea and vomiting, polyuria, polydipsia, weakness, pruritus, and nervousness, potentially with irreversible calcification of soft tissue in the kidney and liver. |
|---|
| Treatment | The treatment of acute accidental overdosage of Calcitriol should consist of general supportive measures. If drug ingestion is discovered within a relatively short time, induction of emesis or gastric lavage may be of benefit in preventing further absorption. If the drug has passed through the stomach, the administration of mineral oil may promote its fecal elimination. Serial serum electrolyte determinations (especially calcium), rate of urinary calcium excretion, and assessment of electrocardiographic abnormalities due to hypercalcemia should be obtained. Such monitoring is critical in patients receiving digitalis. Discontinuation of supplemental calcium and a low-calcium diet are also indicated in accidental overdosage. (13) |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB00136 |
|---|
| HMDB ID | HMDB01903 |
|---|
| PubChem Compound ID | 5280453 |
|---|
| ChEMBL ID | CHEMBL846 |
|---|
| ChemSpider ID | 4444108 |
|---|
| KEGG ID | C01673 |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | 193100 , 259750 , 264700 , 277440 , 601199 |
|---|
| ChEBI ID | 17823 |
|---|
| BioCyc ID | CALCITRIOL |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Calcitriol |
|---|
| PDB ID | VDX |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Calcitriol |
|---|
| References |
|---|
| Synthesis Reference | Raymond E. Conrow, “Process for preparation of calcitriol lactone and related intermediates.” U.S. Patent US5457245, issued April, 1994. |
|---|
| MSDS | Link |
|---|
| General References | - Shepard RM, Horst RL, Hamstra AJ, DeLuca HF: Determination of vitamin D and its metabolites in plasma from normal and anephric man. Biochem J. 1979 Jul 15;182(1):55-69. [227368 ]
- Mason RS, Frankel T, Chan YL, Lissner D, Posen S: Vitamin D conversion by sarcoid lymph node homogenate. Ann Intern Med. 1984 Jan;100(1):59-61. [6546329 ]
- Mallette LE: Case report: hypoparathyroidism with menses-associated hypocalcemia. Am J Med Sci. 1992 Jul;304(1):32-7. [1642251 ]
- Ott SM, Chesnut CH 3rd: Calcitriol treatment is not effective in postmenopausal osteoporosis. Ann Intern Med. 1989 Feb 15;110(4):267-74. [2913914 ]
- Bhan I, Shah A, Holmes J, Isakova T, Gutierrez O, Burnett SM, Juppner H, Wolf M: Post-transplant hypophosphatemia: Tertiary 'Hyper-Phosphatoninism'? Kidney Int. 2006 Oct;70(8):1486-94. Epub 2006 Aug 30. [16941023 ]
- Josephson MA, Schumm LP, Chiu MY, Marshall C, Thistlethwaite JR, Sprague SM: Calcium and calcitriol prophylaxis attenuates posttransplant bone loss. Transplantation. 2004 Oct 27;78(8):1233-6. [15502727 ]
- Gallagher JC, Goldgar D: Treatment of postmenopausal osteoporosis with high doses of synthetic calcitriol. A randomized controlled study. Ann Intern Med. 1990 Nov 1;113(9):649-55. [2221645 ]
- Lehmann B, Sauter W, Knuschke P, Dressler S, Meurer M: Demonstration of UVB-induced synthesis of 1 alpha,25-dihydroxyvitamin D3 (calcitriol) in human skin by microdialysis. Arch Dermatol Res. 2003 Apr;295(1):24-8. Epub 2003 Mar 11. [12709817 ]
- Moreno J, Krishnan AV, Swami S, Nonn L, Peehl DM, Feldman D: Regulation of prostaglandin metabolism by calcitriol attenuates growth stimulation in prostate cancer cells. Cancer Res. 2005 Sep 1;65(17):7917-25. [16140963 ]
- Kalkwarf HJ, Specker BL, Heubi JE, Vieira NE, Yergey AL: Intestinal calcium absorption of women during lactation and after weaning. Am J Clin Nutr. 1996 Apr;63(4):526-31. [8599316 ]
- Mason RS, Lissner D, Grunstein HS, Posen S: A simplified assay for dihydroxylated vitamin D metabolites in human serum: application to hyper- and hypovitaminosis D. Clin Chem. 1980 Mar;26(3):444-50. [6892691 ]
- Drugs.com [Link]
- RxList: The Internet Drug Index (2009). [Link]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | | Gene | Gene Symbol | Gene ID | Interaction | Chromosome | Details |
|---|
|
|---|
| Down-Regulated Genes | | Gene | Gene Symbol | Gene ID | Interaction | Chromosome | Details |
|---|
|
|---|