| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2009-07-21 20:27:32 UTC |
|---|
| Update Date | 2014-12-24 20:25:52 UTC |
|---|
| Accession Number | T3D2876 |
|---|
| Identification |
|---|
| Common Name | Modafinil |
|---|
| Class | Small Molecule |
|---|
| Description | Modafinil is a stimulant drug marketed as a 'wakefulness promoting agent' and is one of the stimulants used in the treatment of narcolepsy. Narcolepsy is caused by dysfunction of a family of wakefulness-promoting and sleep-suppressing peptides, the orexins, whose neurons are activated by modafinil. The prexin neuron activation is associated with psychoactivation and euphoria. The exact mechanism of action is unclear, although in vitro studies have shown it to inhibit the reuptake of dopamine by binding to the dopamine reuptake pump, and lead to an increase in extracellular dopamine. Modafinil activates glutamatergic circuits while inhibiting GABA. |
|---|
| Compound Type | - Amide
- Amine
- Anorexigenic Agent
- Appetite Depressant
- Central Nervous System Agent
- Central Nervous System Stimulant
- Drug
- Metabolite
- Neuroprotective Agent
- Organic Compound
- Stimulant
- Synthetic Compound
|
|---|
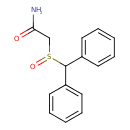
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | Alertec | | Alertex | | Aspendos | | Forcilin | | Mentix | | Modafinilo | | Modafinilum | | Modasomil | | Modavigil | | Moderateafinil | | Modiodal | | Provake | | Provigil | | Resotyl | | Sparlon | | Stavigile | | Vigicer | | Vigil | | Zalux |
|
|---|
| Chemical Formula | C15H15NO2S |
|---|
| Average Molecular Mass | 273.350 g/mol |
|---|
| Monoisotopic Mass | 273.350 g/mol |
|---|
| CAS Registry Number | 68693-11-8 |
|---|
| IUPAC Name | 2-diphenylmethanesulfinylacetamide |
|---|
| Traditional Name | modafinil |
|---|
| SMILES | OC(=N)CS(=O)C(C1=CC=CC=C1)C1=CC=CC=C1 |
|---|
| InChI Identifier | InChI=1/C15H15NO2S/c16-14(17)11-19(18)15(12-7-3-1-4-8-12)13-9-5-2-6-10-13/h1-10,15H,11H2,(H2,16,17) |
|---|
| InChI Key | InChIKey=YFGHCGITMMYXAQ-UHFFFAOYNA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as diphenylmethanes. Diphenylmethanes are compounds containing a diphenylmethane moiety, which consists of a methane wherein two hydrogen atoms are replaced by two phenyl groups. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Diphenylmethanes |
|---|
| Direct Parent | Diphenylmethanes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Diphenylmethane
- Benzyl alkyl sulfoxide
- Benzyl sulfoxide
- Carboxamide group
- Primary carboxylic acid amide
- Sulfoxide
- Sulfinyl compound
- Carboxylic acid derivative
- Hydrocarbon derivative
- Carbonyl group
- Organosulfur compound
- Organooxygen compound
- Organonitrogen compound
- Organic nitrogen compound
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | - Cytoplasm
- Extracellular
- Membrane
|
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | 164-166°C | | Boiling Point | Not Available | | Solubility | Slightly soluble | | LogP | 0.6 |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-014i-2900000000-528a0bbea5cbea3771e1 | 2017-09-01 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 35V, Positive | splash10-014i-0900000000-e85eba164d5df6d6552e | 2021-09-20 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0390000000-c3fbad26370e7ff03677 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0560-1190000000-55b81c4a8b771b4a2bf5 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-02bf-8920000000-9bdc4395d4fc1f10feaa | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-2190000000-a8f51eb41df43ec99388 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-006x-8390000000-595fb55087a29048faca | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-05mo-9500000000-66e73e7a840f3754f786 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0900000000-c692fb05e504a5da303a | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-0900000000-c692fb05e504a5da303a | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014i-0900000000-1fe50a8d9579a6198a36 | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-024i-0190000000-06b6e8449d4102829ac2 | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-7900000000-4df9193e933f8acc3b4b | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-03di-9310000000-7866ba7c4275aa6d34a0 | 2021-10-11 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Rapid following oral administration. |
|---|
| Mechanism of Toxicity | The exact mechanism of action is unclear, although in vitro studies have shown it to inhibit the reuptake of dopamine by binding to the dopamine reuptake pump, and lead to an increase in extracellular dopamine. Modafinil activates glutamatergic circuits while inhibiting GABA. Modafinil is thought to have less potential for abuse than other stimulants due to the absence of any significant euphoric or pleasurable effects. It is possible that modafinil acts by a synergistic combination of mechanisms including direct inhibition of dopamine reuptake, indirect inhibition of noradrenalin reuptake in the VLPO and orexin activation. Modafinil has partial alpha 1B-adrenergic agonist effects by directly stimulating the receptors. |
|---|
| Metabolism | Hepatic
Route of Elimination: The major route of elimination is metabolism (~90%), primarily by the liver, with subsequent renal elimination of the metabolites.
Half Life: 23-215 hours |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | In the U.S, only for the treatment of narcolepsy, obstructive sleep apnea/hypopnea and shift work sleep disorder. In some countries, it is also approved for idiopathic hypersomnia. [Wikipedia] |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Using large amounts of these drugs can result in a condition known as amphetamine psychosis -- which can result in auditory, visual and tactile hallucinations, intense paranoia, irrational thoughts and beliefs, delusions, and mental confusion. Using large amounts of these drugs can result in a condition known as amphetamine psychosis -- which can result in auditory, visual and tactile hallucinations, intense paranoia, irrational thoughts and beliefs, delusions, and mental confusion. |
|---|
| Symptoms | Side-effects can include sweating, dry mouth, blurred vision, insomnia, loss of appetite, and dizziness. In addition users can feel restless, anxious and moody, become excitable and have a false sense of power and security. Amphetamine overdose can also cause cardiac arrhythmias, headaches, convulsions, hypertension, rapid heart rate, coma and death. Amphetamines are psychologically and physically addictive. |
|---|
| Treatment | No specific antidote to the toxic effects of modafinil overdose has been identified to date. Such overdoses should be managed with primarily supportive care, including cardiovascular monitoring. If there are no contraindications, induced emesis or gastric lavage should be considered. (4) |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB00745 |
|---|
| HMDB ID | HMDB14883 |
|---|
| PubChem Compound ID | 4236 |
|---|
| ChEMBL ID | CHEMBL1373 |
|---|
| ChemSpider ID | 4088 |
|---|
| KEGG ID | Not Available |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | 417124 |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Modafinil |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Modafinil |
|---|
| References |
|---|
| Synthesis Reference | DrugSyn.org |
|---|
| MSDS | Link |
|---|
| General References | - Lindsay SE, Gudelsky GA, Heaton PC: Use of modafinil for the treatment of attention deficit/hyperactivity disorder. Ann Pharmacother. 2006 Oct;40(10):1829-33. Epub 2006 Sep 5. [16954326 ]
- Ishizuka T, Sakamoto Y, Sakurai T, Yamatodani A: Modafinil increases histamine release in the anterior hypothalamus of rats. Neurosci Lett. 2003 Mar 20;339(2):143-6. [12614915 ]
- Drugs.com [Link]
- RxList: The Internet Drug Index (2009). [Link]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|