| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2009-07-21 20:27:59 UTC |
|---|
| Update Date | 2014-12-24 20:25:53 UTC |
|---|
| Accession Number | T3D2935 |
|---|
| Identification |
|---|
| Common Name | Diethylpropion |
|---|
| Class | Small Molecule |
|---|
| Description | Diethylpropion is only found in individuals that have used or taken this drug. It is a appetite depressant considered to produce less central nervous system disturbance than most drugs in this therapeutic category. It is also considered to be among the safest for patients with hypertension. (From AMA Drug Evaluations Annual, 1994, p2290)Diethylpropion is an amphetamine that stimulates neurons to release or maintain high levels of a particular group of neurotransmitters known as catecholamines; these include dopamine and norepinephrine. High levels of these catecholamines tend to suppress hunger signals and appetite. Diethylpropion (through catecholamine elevation) may also indirectly affect leptin levels in the brain. It is theorized that diethylpropion can raise levels of leptin which signal satiety. It is also theorized that increased levels of the catecholamines are partially responsible for halting another chemical messenger known as neuropeptide Y. This peptide initiates eating, decreases energy expenditure, and increases fat storage. |
|---|
| Compound Type | - Amine
- Anorexigenic Agent
- Appetite Depressant
- Drug
- Ester
- Metabolite
- Organic Compound
- Stimulant
- Synthetic Compound
|
|---|
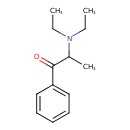
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | (+-)-Diethylpropion | | (±)-diethylpropion | | 1-phenyl-2-diethylamino-1-propanone | | 2-(Diethylamino)-1-phenyl-1-propanone | | 2-(Diethylamino)propiophenone | | alpha-Benzoyltriethylamine | | alpha-Diethylaminopropiophenone | | Amfepramone | | Amfepramonum | | Anfepramona | | Anorex | | DEA No. 1610 | | Diethylcathinone | | Diethylpropione | | Dobesin | | Frekentine | | Linea | | Moderatan | | Neobes | | Nobesine | | Prefamone | | Regenon | | Tenuate | | Tepanil | | Tylinal |
|
|---|
| Chemical Formula | C13H19NO |
|---|
| Average Molecular Mass | 205.296 g/mol |
|---|
| Monoisotopic Mass | 205.147 g/mol |
|---|
| CAS Registry Number | 90-84-6 |
|---|
| IUPAC Name | 2-(diethylamino)-1-phenylpropan-1-one |
|---|
| Traditional Name | diethylpropion |
|---|
| SMILES | CCN(CC)C(C)C(=O)C1=CC=CC=C1 |
|---|
| InChI Identifier | InChI=1/C13H19NO/c1-4-14(5-2)11(3)13(15)12-9-7-6-8-10-12/h6-11H,4-5H2,1-3H3 |
|---|
| InChI Key | InChIKey=XXEPPPIWZFICOJ-UHFFFAOYNA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as alkyl-phenylketones. These are aromatic compounds containing a ketone substituted by one alkyl group, and a phenyl group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbonyl compounds |
|---|
| Direct Parent | Alkyl-phenylketones |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alkyl-phenylketone
- Phenylpropane
- Benzoyl
- Aryl alkyl ketone
- Benzenoid
- Monocyclic benzene moiety
- Alpha-aminoketone
- Tertiary aliphatic amine
- Tertiary amine
- Hydrocarbon derivative
- Amine
- Organic nitrogen compound
- Organonitrogen compound
- Organopnictogen compound
- Organic oxide
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | 168°C | | Boiling Point | Not Available | | Solubility | 1.22e+00 g/L | | LogP | 2.8 |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0udi-9600000000-6ee5a640521355e9e385 | 2017-09-12 | View Spectrum | | GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0udi-9600000000-6ee5a640521355e9e385 | 2018-05-18 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0pb9-4900000000-dfe406cfb26414d42c91 | 2017-09-01 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0390000000-ce898493104c3b4c2c47 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0pb9-1920000000-96569e0397bae3f7dad7 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0kor-9400000000-099deea1407beaa58a50 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0190000000-95dcba361e53234bdef9 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-5590000000-1b565cac019e1aaf4095 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0kl0-8900000000-7a17ddfd7a2fe78351c6 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0290000000-e6b4a514d0501abfde52 | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0zfr-1920000000-454738ae8af99f176428 | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0fb9-9500000000-1d8dda4d3070e4f07ca5 | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0090000000-cd886772587c719fa7c6 | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0ufr-6690000000-31723177ed3fe7247254 | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-9100000000-96fa4edb3fe7918f8909 | 2021-10-11 | View Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-0udi-2900000000-43214c102b76f59ba4d1 | 2014-09-20 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Diethylpropion is rapidly absorbed from the GI tract after oral administration. |
|---|
| Mechanism of Toxicity | Diethylpropion is an amphetamine that stimulates neurons to release or maintain high levels of a particular group of neurotransmitters known as catecholamines; these include dopamine and norepinephrine. High levels of these catecholamines tend to suppress hunger signals and appetite. Diethylpropion (through catecholamine elevation) may also indirectly affect leptin levels in the brain. It is theorized that diethylpropion can raise levels of leptin which signal satiety. It is also theorized that increased levels of the catecholamines are partially responsible for halting another chemical messenger known as neuropeptide Y. This peptide initiates eating, decreases energy expenditure, and increases fat storage. |
|---|
| Metabolism | Extensively metabolized through a complex pathway of biotransformation involving N-dealkylation and reduction. Many of these metabolites are biologically active and may participate in the therapeutic action of diethylpropion.
Route of Elimination: Diethylpropion is rapidly absorbed from the GI tract after oral administration and is extensively metabolized through a complex pathway of biotransformation involving N-dealkylation and reduction. Diethylpropion and/or its active metabolites are believed to cross the blood-brain barrier and the placenta. Diethylpropion and its metabolites are excreted mainly by the kidney.
Half Life: Using a phosphorescence assay that is specific for basic compounds containing benzoyl group, the plasma half-life of the aminoketone metabolites is estimated to be between 4 to 6 hours. |
|---|
| Toxicity Values | LD50: 600 mg/kg (Oral, Mouse) (1)
LD50: 50 mg/kg (Oral, Rat) (1)
LD50: 225 mg/kg (Oral, Dog) (1) |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | Used as an antidepressant. [Wikipedia]. Used in the management of exogenous obesity as a short-term adjunct (a few weeks) in a regimen of weight reduction based on caloric restriction. |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Using large amounts of these drugs can result in a condition known as amphetamine psychosis -- which can result in auditory, visual and tactile hallucinations, intense paranoia, irrational thoughts and beliefs, delusions, and mental confusion. |
|---|
| Symptoms | Manifestation of acute overdosage include restlessness, tremor, hyperreflexia, rapid respiration, confusion, assaultiveness, hallucinations, and panic states. |
|---|
| Treatment | Management of acute diethylpropion hydrochloride intoxication is largely symptomatic and includes lavage and sedation with a barbiturate. Experience with hemodialysis or peritoneal dialysis is inadequate to permit recommendation in this regard. Intravenous phentolamine (Regitine |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB00937 |
|---|
| HMDB ID | HMDB15072 |
|---|
| PubChem Compound ID | 7029 |
|---|
| ChEMBL ID | CHEMBL1194666 |
|---|
| ChemSpider ID | 6762 |
|---|
| KEGG ID | C06954 |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | 4530 |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Diethylpropion |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Diethylpropion |
|---|
| References |
|---|
| Synthesis Reference | Schutte, J.; U.S. Patent 3,001,910; September 26, 1961; assigned to Firma Ternmler-Werke
(W. Germany). |
|---|
| MSDS | Link |
|---|
| General References | - Wishart DS, Knox C, Guo AC, Cheng D, Shrivastava S, Tzur D, Gautam B, Hassanali M: DrugBank: a knowledgebase for drugs, drug actions and drug targets. Nucleic Acids Res. 2008 Jan;36(Database issue):D901-6. Epub 2007 Nov 29. [18048412 ]
- Adan RA, Vanderschuren LJ, la Fleur SE: Anti-obesity drugs and neural circuits of feeding. Trends Pharmacol Sci. 2008 Apr;29(4):208-17. doi: 10.1016/j.tips.2008.01.008. Epub 2008 Mar 18. [18353447 ]
- Arias HR, Santamaria A, Ali SF: Pharmacological and neurotoxicological actions mediated by bupropion and diethylpropion. Int Rev Neurobiol. 2009;88:223-55. doi: 10.1016/S0074-7742(09)88009-4. [19897080 ]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|