| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2009-07-21 20:28:03 UTC |
|---|
| Update Date | 2014-12-24 20:25:53 UTC |
|---|
| Accession Number | T3D2942 |
|---|
| Identification |
|---|
| Common Name | Mepivacaine |
|---|
| Class | Small Molecule |
|---|
| Description | A local anesthetic that is chemically related to bupivacaine but pharmacologically related to lidocaine. It is indicated for infiltration, nerve block, and epidural anesthesia. Mepivacaine is effective topically only in large doses and therefore should not be used by this route. (From AMA Drug Evaluations, 1994, p168) |
|---|
| Compound Type | - Amide
- Amine
- Anesthetic, Local
- Drug
- Metabolite
- Organic Compound
- Synthetic Compound
|
|---|
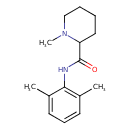
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | (+-)-1-Methyl-2',6'-pipecoloxylidide | | 1-methyl-2',6'-pipecoloxylidide | | Carbocaine | | DL-Mepivacaine | | Isocaine | | Mepivacaina | | Mepivacaine HCL | | Mepivacaine hydrochloride | | Mepivacainum | | Mepivicaine | | N-(2,6-Dimethylphenyl)-1-methyl-2-piperidinecarboxamide | | N-(2,6-Dimethylphenyl)-1-methylpiperidine-2-carboxamide | | Polocaine | | S-Ropivacaine Mesylate | | Scandicaine | | Scandonest Plain |
|
|---|
| Chemical Formula | C15H22N2O |
|---|
| Average Molecular Mass | 246.348 g/mol |
|---|
| Monoisotopic Mass | 246.173 g/mol |
|---|
| CAS Registry Number | 96-88-8 |
|---|
| IUPAC Name | N-(2,6-dimethylphenyl)-1-methylpiperidine-2-carboxamide |
|---|
| Traditional Name | mepivacaine |
|---|
| SMILES | CN1CCCCC1C(O)=NC1=C(C)C=CC=C1C |
|---|
| InChI Identifier | InChI=1/C15H22N2O/c1-11-7-6-8-12(2)14(11)16-15(18)13-9-4-5-10-17(13)3/h6-8,13H,4-5,9-10H2,1-3H3,(H,16,18) |
|---|
| InChI Key | InChIKey=INWLQCZOYSRPNW-UHFFFAOYNA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as piperidinecarboxamides. Piperidinecarboxamides are compounds containing a piperidine ring substituted with a carboxamide functional group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Piperidines |
|---|
| Sub Class | Piperidinecarboxylic acids and derivatives |
|---|
| Direct Parent | Piperidinecarboxamides |
|---|
| Alternative Parents | |
|---|
| Substituents | - 2-piperidinecarboxamide
- Piperidinecarboxamide
- M-xylene
- Xylene
- Monocyclic benzene moiety
- Benzenoid
- Tertiary aliphatic amine
- Tertiary amine
- Carboximidic acid
- Carboximidic acid derivative
- Azacycle
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Amine
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Organopnictogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | - Cytoplasm
- Extracellular
- Membrane
|
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | | Name | SMPDB Link | KEGG Link |
|---|
| Mepivacaine Pathway | Not Available | Not Available |
|
|---|
| Applications | |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | 150.5°C | | Boiling Point | Not Available | | Solubility | 7000 mg/L (at 23°C) | | LogP | 1.95 |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0092-9200000000-a3a931af35da130669aa | 2017-09-01 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , positive | splash10-0002-9040000000-3c0a89a13f39484eef97 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , positive | splash10-0002-9000000000-9073826aa2631981fe72 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , positive | splash10-0002-9000000000-9b273b91b5148ef6a8fa | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , positive | splash10-0002-9000000000-d541d397ca20f2f1a799 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , positive | splash10-0002-9000000000-eda45d03ea77058a8429 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , positive | splash10-006t-9000000000-f42a147676c3c718e38f | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 30V, Positive | splash10-0002-9000000000-9073826aa2631981fe72 | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 45V, Positive | splash10-0002-9000000000-9b273b91b5148ef6a8fa | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 15V, Positive | splash10-0002-9040000000-3c0a89a13f39484eef97 | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 60V, Positive | splash10-0002-9000000000-d541d397ca20f2f1a799 | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 75V, Positive | splash10-0002-9000000000-4b9a3b3048a6c1bbd656 | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 90V, Positive | splash10-006t-9000000000-f42a147676c3c718e38f | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 35V, Positive | splash10-0002-9000000000-e7a7508a46c81f01a0f6 | 2021-09-20 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-006t-2890000000-4fd4344949c8e98d2d22 | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-006t-9600000000-eccb3e339a3aafb57c83 | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00di-9100000000-923210fdcbf3093cec8c | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0190000000-5c29854d995646f8e15f | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00fs-0960000000-75e4d525847b240eb8dd | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0g4i-6900000000-79da7c9893bedd422f3e | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-9380000000-6935f06f321d22f49f8f | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-9630000000-fc9251019b6aa4d95ac0 | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0002-9000000000-64648fb1fd5128b76827 | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0090000000-4a0996a0879be85733ed | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00dj-0950000000-7a84cb81cb305d4c644e | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00xr-1910000000-dcbc91df93a09683c261 | 2021-10-11 | View Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-0002-9000000000-6a3d80d214452a24ab62 | 2014-09-20 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Absorbed locally. The rate of systemic absorption of local anesthetics is dependent upon the total dose and concentration of drug administered, the route of administration, the vascularity of the administration site, and the presence or absence of epinephrine in the anesthetic solution. Subcutaneous, injection ; Infiltration |
|---|
| Mechanism of Toxicity | Local anesthetics block the generation and the conduction of nerve impulses, presumably by increasing the threshold for electrical excitation in the nerve, by slowing the propagation of the nerve impulse, and by reducing the rate of rise of the action potential. In general, the progression of anesthesia is related to the diameter, myelination, and conduction velocity of affected nerve fibers. Clinically, the order of loss of nerve function is as follows: pain, temperature, touch, proprioception, and skeletal muscle tone. |
|---|
| Metabolism | Rapidly metabolized, with only a small percentage of the anesthetic (5 percent to 10 percent) being excreted unchanged in the urine. The liver is the principal site of metabolism, with over 50% of the administered dose being excreted into the bile as metabolites.
Route of Elimination: It is rapidly metabolized, with only a small percentage of the anesthetic (5 percent to 10 percent) being excreted unchanged in the urine.The liver is the principal site of metabolism, with over 50% of the administered dose being excreted into the bile as metabolites.
Half Life: The half-life of mepivacaine in adults is 1.9 to 3.2 hours and in neonates 8.7 to 9 hours. |
|---|
| Toxicity Values | The mean seizure dosage of mepivacaine in rhesus monkeys was found to be 18.8 mg/kg with mean arterial plasma concentration of 24.4 µg/mL.
LD50: 23-35 mg/kg (Intravenous, Mouse) (1)
LD50: 280 mg/kg (Subcutaneous, Mouse (1) |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | For production of local or regional analgesia and anesthesia by local infiltration, peripheral nerve block techniques, and central neural techniques including epidural and caudal blocks. |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Treatment of a patient with toxic manifestations consists of assuring and maintaining a patient airway and supporting ventilation (respiration) as required. This usually will be sufficient in the management of most reactions. Should a convulsion persist despite ventilatory therapy, small increments of anticonvulsive agents may be given intravenously, such as benzodiazephine (e.g., diazepam) or ultrashort-acting barbiturates (e.g., thiopental or thiamylal) or short-acting barbiturates (e.g., pentobarbital or secobarbital). Cardiovascular depression may require circulatory assistance with intravenous fluids and/or vasopressor (e.g., Ephedrine) as dictated by the clinical situation. (4) |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB00961 |
|---|
| HMDB ID | HMDB15096 |
|---|
| PubChem Compound ID | 4062 |
|---|
| ChEMBL ID | CHEMBL1087 |
|---|
| ChemSpider ID | 3922 |
|---|
| KEGG ID | C07528 |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | 6759 |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Mepivacaine |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Mepivacaine |
|---|
| References |
|---|
| Synthesis Reference | DrugSyn.org |
|---|
| MSDS | Link |
|---|
| General References | - Wishart DS, Knox C, Guo AC, Cheng D, Shrivastava S, Tzur D, Gautam B, Hassanali M: DrugBank: a knowledgebase for drugs, drug actions and drug targets. Nucleic Acids Res. 2008 Jan;36(Database issue):D901-6. Epub 2007 Nov 29. [18048412 ]
- Driessen B, Reimann W: Interaction of the central analgesic, tramadol, with the uptake and release of 5-hydroxytryptamine in the rat brain in vitro. Br J Pharmacol. 1992 Jan;105(1):147-51. [1596676 ]
- AMA Drug Evaluations, 1994, p168.
- RxList: The Internet Drug Index (2009). [Link]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|