| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2009-07-21 20:28:37 UTC |
|---|
| Update Date | 2014-12-24 20:25:55 UTC |
|---|
| Accession Number | T3D3018 |
|---|
| Identification |
|---|
| Common Name | Rifapentine |
|---|
| Class | Small Molecule |
|---|
| Description | Rifapentine is only found in individuals that have used or taken this drug. It is an antibiotic drug used in the treatment of tuberculosis. Rifapentine has shown higher bacteriostatic and bactericidal activities especially against intracellular bacteria growing in human monocyte-derived macrophages. Rifapentine inhibits DNA-dependent RNA polymerase in susceptible strains of M. tuberculosis. Rifapentine acts via the inhibition of DNA-dependent RNA polymerase, leading to a suppression of RNA synthesis and cell death. |
|---|
| Compound Type | - Amide
- Amine
- Antibiotic, Antitubercular
- Antimycobacterial
- Drug
- Ester
- Ether
- Hydrazine
- Leprostatic Agent
- Metabolite
- Organic Compound
- Synthetic Compound
|
|---|
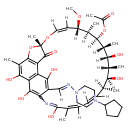
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | 3-(((4-Cyclopentyl-1-piperazinyl)imino)methyl)rifamycin | | Cyclopentyl rifampin | | Cyclopentylrifampicin | | Priftin |
|
|---|
| Chemical Formula | C47H64N4O12 |
|---|
| Average Molecular Mass | 877.031 g/mol |
|---|
| Monoisotopic Mass | 876.452 g/mol |
|---|
| CAS Registry Number | 61379-65-5 |
|---|
| IUPAC Name | (7S,9Z,11S,12R,13S,14R,15R,16R,17S,18S,21Z)-26-[(1E)-[(4-cyclopentylpiperazin-1-yl)imino]methyl]-2,15,17,23,27,29-hexahydroxy-11-methoxy-3,7,12,14,16,18,22-heptamethyl-6-oxo-8,30-dioxa-24-azatetracyclo[23.3.1.1^{4,7}.0^{5,28}]triaconta-1(28),2,4,9,19,21,23,25(29),26-nonaen-13-yl acetate |
|---|
| Traditional Name | (7S,9Z,11S,12R,13S,14R,15R,16R,17S,18S,21Z)-26-[(1E)-[(4-cyclopentylpiperazin-1-yl)imino]methyl]-2,15,17,23,27,29-hexahydroxy-11-methoxy-3,7,12,14,16,18,22-heptamethyl-6-oxo-8,30-dioxa-24-azatetracyclo[23.3.1.1^{4,7}.0^{5,28}]triaconta-1(28),2,4,9,19,21,23,25(29),26-nonaen-13-yl acetate |
|---|
| SMILES | [H]\C(=N/N1CCN(CC1)C1CCCC1)C1=C(O)C2=C3C(O)=C(C)C4=C2C(=O)[C@](C)(O4)O\C([H])=C([H])/[C@]([H])(OC)[C@@]([H])(C)[C@@]([H])(OC(C)=O)[C@]([H])(C)[C@]([H])(O)[C@]([H])(C)[C@@]([H])(O)[C@@]([H])(C)C([H])=C([H])\C([H])=C(C)/C(O)=NC1=C3O |
|---|
| InChI Identifier | InChI=1S/C47H64N4O12/c1-24-13-12-14-25(2)46(59)49-37-32(23-48-51-20-18-50(19-21-51)31-15-10-11-16-31)41(56)34-35(42(37)57)40(55)29(6)44-36(34)45(58)47(8,63-44)61-22-17-33(60-9)26(3)43(62-30(7)52)28(5)39(54)27(4)38(24)53/h12-14,17,22-24,26-28,31,33,38-39,43,53-57H,10-11,15-16,18-21H2,1-9H3,(H,49,59)/b13-12+,22-17-,25-14-,48-23+/t24-,26+,27+,28+,33-,38-,39+,43+,47-/m0/s1 |
|---|
| InChI Key | InChIKey=WDZCUPBHRAEYDL-IWVAIHGYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as naphthofurans. Naphthofurans are compounds containing a furan ring fused to a naphthalene moiety. Furan is a 5 membered- ring aromatic ring with four carbon and one oxygen atoms. Naphthalene is a polycyclic aromatic hydrocarbon made up of two fused benzene rings. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Naphthofurans |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Naphthofurans |
|---|
| Alternative Parents | Not Available |
|---|
| Substituents | Not Available |
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | |
|---|
| Biological Roles | |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | 2.13e-02 g/L | | LogP | 4 |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0aor-0300000190-d118c1ac8e3c60e6452b | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014l-2100002290-b441d6ad4afc88a62624 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014l-9700006020-ee8161658ea49259d168 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0100001290-6aa51e29ae4edc2dea4f | 2016-08-04 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-066r-2500003190-ac076b0cb13b991b003a | 2016-08-04 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a6r-9200008320-465071920392bca6a779 | 2016-08-04 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-056r-0000000390-f812402cd842a853749c | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0690-0000000190-f6ddb4327cad4885918e | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0cfr-7600001490-17f0e57624f1de4d2dee | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-1000000290-3b4cef67ccc26d401a39 | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-9000000010-4e966171b9c3614260a0 | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4m-9500006520-7a1bb51257442c63c784 | 2021-10-11 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Rapidly and well absorbed from the gastrointestinal tract. |
|---|
| Mechanism of Toxicity | Rifapentine has shown higher bacteriostatic and bactericidal activities especially against intracellular bacteria growing in human monocyte-derived macrophages. Rifapentine inhibits DNA-dependent RNA polymerase in susceptible strains of M. tuberculosis. Rifapentine acts via the inhibition of DNA-dependent RNA polymerase, leading to a suppression of RNA synthesis and cell death. |
|---|
| Metabolism | Hepatic
Route of Elimination: Following a single 600 mg oral dose of radiolabeled rifapentine to healthy volunteers (n=4), 87% of the total 14C rifapentine was recovered in the urine (17%) and feces (70%). |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | For the treatment of pulmonary tuberculosis. |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB01201 |
|---|
| HMDB ID | HMDB15332 |
|---|
| PubChem Compound ID | 6323497 |
|---|
| ChEMBL ID | CHEMBL1660 |
|---|
| ChemSpider ID | 4883449 |
|---|
| KEGG ID | C08059 |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | 8861 |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Rifapentine |
|---|
| PDB ID | RPT |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Rifapentine |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Link |
|---|
| General References | - Drugs.com [Link]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | | Gene | Gene Symbol | Gene ID | Interaction | Chromosome | Details |
|---|
|
|---|
| Down-Regulated Genes | Not Available |
|---|