| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2009-07-30 17:58:32 UTC |
|---|
| Update Date | 2014-12-24 20:26:06 UTC |
|---|
| Accession Number | T3D3468 |
|---|
| Identification |
|---|
| Common Name | Isopropylbenzene |
|---|
| Class | Small Molecule |
|---|
| Description | Isopropylbenzene is found in ceylan cinnamon. Isopropylbenzene is a trace constituent of ginger oil (Zingiber officinale) Cumene is the common name for isopropylbenzene, an organic compound that is an aromatic hydrocarbon. It is a constituent of crude oil and refined fuels. It is a flammable colorless liquid that has a boiling point of 152 C. Nearly all the cumene that is produced as a pure compound on an industrial scale is converted to cumene hydroperoxide, which is an intermediate in the synthesis of other industrially important chemicals such as phenol and acetone.
Isopropylbenzene has been shown to exhibit catabolic function (3). |
|---|
| Compound Type | - Food Toxin
- Gasoline Additive/Component
- Household Toxin
- Industrial/Workplace Toxin
- Metabolite
- Natural Compound
- Organic Compound
- Pollutant
|
|---|
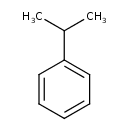
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | (1-methylethyl)-Benzene | | (1-Methylethyl)benzene | | (1-methylethyl)benzene (cumene) | | (methylethyl)benzene | | (propan-2-yl)benzene | | 1-Methylethyl-Benzene | | 1-Methylethylbenzene, 9CI | | 2-Fenilpropano | | 2-Fenyl-propaan | | 2-Phenyl-Propane | | 2-Phenylpropane | | Benzene, (1-methylethyl)-, oxidized, polyphenyl residues | | Benzene, (1-methylethyl)-, oxidized, sulfurized by-products | | Benzene, isopropyl | | Benzene,isopropyl cumol | | Cumeen | | Cumene | | CUMENE (CUMENE HYDROPEROXIDE (80-15-9)) | | Cumol | | I-Propyl-Benzene | | I-Propylbenzene | | Iso-propylbenzene (cumene) | | Isopropilbenzene | | Isopropyl-Benzene | | Isopropyl-benzol | | Isopropylbenzeen | | Isopropylbenzen | | Isopropylbenzol | | Oxidized cumene polyphenyl residues | | Phenol bottoms | | Polyphenyl residue | | Propane, 2-phenyl | | Sulfurized BY-product of cumene oxidation |
|
|---|
| Chemical Formula | C9H12 |
|---|
| Average Molecular Mass | 120.192 g/mol |
|---|
| Monoisotopic Mass | 120.094 g/mol |
|---|
| CAS Registry Number | 98-82-8 |
|---|
| IUPAC Name | (propan-2-yl)benzene |
|---|
| Traditional Name | cumene |
|---|
| SMILES | CC(C)C1=CC=CC=C1 |
|---|
| InChI Identifier | InChI=1S/C9H12/c1-8(2)9-6-4-3-5-7-9/h3-8H,1-2H3 |
|---|
| InChI Key | InChIKey=RWGFKTVRMDUZSP-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as cumenes. These are aromatic compounds containing a prop-2-ylbenzene moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Cumenes |
|---|
| Direct Parent | Cumenes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Phenylpropane
- Cumene
- Aromatic hydrocarbon
- Unsaturated hydrocarbon
- Hydrocarbon
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Liquid |
|---|
| Appearance | Colorless liquid. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | -96.9°C | | Boiling Point | Not Available | | Solubility | 0.0613 mg/mL at 25°C | | LogP | 3.66 |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0a4i-5900000000-d7826e94822da05cb9c2 | 2017-09-12 | View Spectrum | | GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0a4i-5900000000-656039b1dd961fda4904 | 2017-09-12 | View Spectrum | | GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0a4i-5900000000-b19bd34b1ebead05ac65 | 2017-09-12 | View Spectrum | | GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0a4i-1900000000-97ac180f6d1c8c215ca1 | 2017-09-12 | View Spectrum | | GC-MS | GC-MS Spectrum - CI-B (Non-derivatized) | splash10-05fr-2900000000-39c80e40c5435cf05394 | 2017-09-12 | View Spectrum | | GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0a4i-5900000000-d7826e94822da05cb9c2 | 2018-05-18 | View Spectrum | | GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0a4i-5900000000-656039b1dd961fda4904 | 2018-05-18 | View Spectrum | | GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0a4i-5900000000-b19bd34b1ebead05ac65 | 2018-05-18 | View Spectrum | | GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0a4i-1900000000-97ac180f6d1c8c215ca1 | 2018-05-18 | View Spectrum | | GC-MS | GC-MS Spectrum - CI-B (Non-derivatized) | splash10-05fr-2900000000-39c80e40c5435cf05394 | 2018-05-18 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0a4i-9800000000-4282a3b73324784a4088 | 2016-09-22 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0900000000-de6900b5ecae080c15b2 | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-2900000000-280ab4235a4dffc8044f | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0pi3-9400000000-58e470689f0010348e80 | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0900000000-8bb4d370a97717061754 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-0900000000-92886075d0b76e117cc7 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014i-5900000000-375849fd3939bf16ab91 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00dl-7900000000-835f64dfcaef3b8ebf30 | 2021-09-24 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004l-9300000000-278676596fcf4bda2bb5 | 2021-09-24 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-004i-9000000000-557a5342da32a2dd8a61 | 2021-09-24 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0900000000-794ffb5dd5f7d8f3ef50 | 2021-09-24 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-0900000000-28d8a72cb103dbe31379 | 2021-09-24 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-9100000000-b5563824f402728d2a26 | 2021-09-24 | View Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-0a4i-7900000000-34bd38a75dcd3bb2fb0e | 2014-09-20 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 400 MHz, CDCl3, experimental) | Not Available | 2014-09-20 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 25.16 MHz, CDCl3, experimental) | Not Available | 2014-09-23 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Oral (10) ; inhalation (10) ; dermal (10) |
|---|
| Mechanism of Toxicity | Petroleum distillates are central nervous system depressants and cause pulmonary damage. (1) |
|---|
| Metabolism | Volatile hydrocarbons are absorbed mainly through the lungs, and may also enter the body after ingestion via aspiration. (1) |
|---|
| Toxicity Values | LD50: 1400 mg/kg (Oral, Rat) (4)
LC50: 24 700 mg/m3 over 2 hours (Inhalation, Mouse) (4) |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | 2B, possibly carcinogenic to humans. (11) |
|---|
| Uses/Sources | Isopropylbenzene is a component of gasoline. Nearly all the cumene that is produced as a pure compound on an industrial scale is converted to cumene hydroperoxide, which is an intermediate in the synthesis of other industrially important chemicals such as phenol and acetone. (9) |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Petroleum distillates are aspiration hazards and may cause pulmonary damage, central nervous system depression, and cardiac effects such as cardiac arrhythmias. They may also affect the blood, immune system, liver, and kidney. (1, 8) |
|---|
| Symptoms | Petroleum distillate poisoning may cause nausea, vomiting, cough, pulmonary irritation progressing to pulmonary edema, bloody sputum, and bronchial pneumonia. At high amounts, central nervous system depression may also occur, with symptoms such as weakness, dizziness, slow and shallow respiration, unconsciousness, and convulsions. Petroleum distillates are also irritating to the skin. (2) |
|---|
| Treatment | Treatment is mainly symptomatic and supportive. Gastric lavage, emesis, and the administration of activated charcoal should be avoided, as vomiting increases the risk of aspiration. (1) |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB34029 |
|---|
| PubChem Compound ID | 7406 |
|---|
| ChEMBL ID | CHEMBL1398949 |
|---|
| ChemSpider ID | 7128 |
|---|
| KEGG ID | C14396 |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | 34656 |
|---|
| BioCyc ID | CPD-1125 |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Isopropylbenzene |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | 1773 |
|---|
| Wikipedia Link | Isopropylbenzene |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | T3D3468.pdf |
|---|
| General References | - Gunther S, McMillan PJ, Wallace LJ, Muller S: Plasmodium falciparum possesses organelle-specific alpha-keto acid dehydrogenase complexes and lipoylation pathways. Biochem Soc Trans. 2005 Nov;33(Pt 5):977-80. [16246025 ]
- Perham RN: Swinging arms and swinging domains in multifunctional enzymes: catalytic machines for multistep reactions. Annu Rev Biochem. 2000;69:961-1004. [10966480 ]
- Eaton RW, Timmis KN: Characterization of a plasmid-specified pathway for catabolism of isopropylbenzene in Pseudomonas putida RE204. J Bacteriol. 1986 Oct;168(1):123-31. [3019995 ]
- Lewis RJ (1996). Sax's Dangerous Properties of Industrial Materials. 9th ed. Volumes 1-3. New York, NY: Van Nostrand Reinhold.
- Dreisbach, RH (1983). Handbook of Poisoning. Los Altos, California: Lange Medical Publications.
- MICROMEDEX Thomson Health Care (2002). USPDI - Drug Information for the Health Care Professional. 22nd ed. Volume 1. Englewood, CO: MICROMEDEX Thomson Health Care. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc.
- Yannai, Shmuel. (2004) Dictionary of food compounds with CD-ROM: Additives, flavors, and ingredients. Boca Raton: Chapman & Hall/CRC.
- ATSDR - Agency for Toxic Substances and Disease Registry (1999). Toxicological profile for total petroleum hydrocarbons (TPH). U.S. Public Health Service in collaboration with U.S. [Link]
- Wikipedia. Cumene. Last Updated 21 May 2009. [Link]
- Wikipedia. Lead telluride. Last Updated 8 May 2009. [Link]
- International Agency for Research on Cancer (2014). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. [Link]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|