| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2009-07-30 17:58:34 UTC |
|---|
| Update Date | 2014-12-24 20:26:06 UTC |
|---|
| Accession Number | T3D3471 |
|---|
| Identification |
|---|
| Common Name | Acetaldehyde |
|---|
| Class | Small Molecule |
|---|
| Description | Acetaldehyde is a colorless, flammable liquid used in the manufacture of acetic acid, perfumes, and flavors. In the chemical industry, acetaldehyde is used as an intermediate in the production of acetic acid, certain esters, and a number of other chemicals. it is also an air pollutant resulting from combustion, such as automotive exhaust and tobacco smoke. It is also an intermediate in the metabolism of alcohol. It has a general narcotic action and also causes irritation of mucous membranes. Large doses may cause death from respiratory paralysis. Small amounts of acetaldehyde are produced naturally through gut microbial fermentation. Acetaldehyde is produced through the action of alcohol dehydrogenase on ethanol and is somewhate more toxic than ethanol. Acetaldehyde is linked to most of the negative clinical effects of alcohol. It has been shown to increase the risk of developing cirrhosis of the liver, multiple forms of cancer, and alcoholism. |
|---|
| Compound Type | - Aldehyde
- Cigarette Toxin
- Food Toxin
- Household Toxin
- Industrial Precursor/Intermediate
- Industrial/Workplace Toxin
- Metabolite
- Natural Compound
- Organic Compound
- Plant Toxin
- Pollutant
|
|---|
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | Acetic aldehyde | | Aldehyde | | Ethanal | | Ethyl aldehyde |
|
|---|
| Chemical Formula | C2H4O |
|---|
| Average Molecular Mass | 44.053 g/mol |
|---|
| Monoisotopic Mass | 44.026 g/mol |
|---|
| CAS Registry Number | 75-07-0 |
|---|
| IUPAC Name | acetaldehyde |
|---|
| Traditional Name | acetaldehyde |
|---|
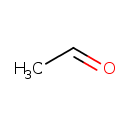
| SMILES | CC=O |
|---|
| InChI Identifier | InChI=1S/C2H4O/c1-2-3/h2H,1H3 |
|---|
| InChI Key | InChIKey=IKHGUXGNUITLKF-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as short-chain aldehydes. These are an aldehyde with a chain length containing between 2 and 5 carbon atoms. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbonyl compounds |
|---|
| Direct Parent | Short-chain aldehydes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Organic oxide
- Hydrocarbon derivative
- Short-chain aldehyde
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Endogenous |
|---|
| Cellular Locations | - Cytoplasm
- Endoplasmic reticulum
- Extracellular
- Mitochondria
- Peroxisome
|
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | - Adrenal Medulla
- Brain
- Erythrocyte
- Fibroblasts
- Gonads
- Intestine
- Kidney
- Liver
- Muscle
- Myelin
- Nerve Cells
- Neuron
- Pancreas
- Placenta
- Platelet
- Stratum Corneum
- Testes
- Thyroid Gland
|
|---|
| Pathways | |
|---|
| Applications | Not Available |
|---|
| Biological Roles | |
|---|
| Chemical Roles | |
|---|
| Physical Properties |
|---|
| State | Liquid |
|---|
| Appearance | Colorless liquid. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | -123°C | | Boiling Point | 21°C (69.8°F) | | Solubility | 1000 mg/mL at 25°C [RIDDICK,JA et al. (1986)] | | LogP | -0.34 |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0006-9000000000-69e31ccd415894a68912 | 2016-09-22 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-0002-9000000000-f1274d4b6066776ca898 | 2012-07-24 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-001l-9000000000-c1e37abbf2ad6054dc10 | 2012-07-24 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-000t-9000000000-2289ead4f7210282cd87 | 2012-07-24 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-9000000000-cf54221d95714f5478c4 | 2015-05-27 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-9000000000-8d8afe7422ae76f7ebb9 | 2015-05-27 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-004j-9000000000-d68dec9f846cfe9acc72 | 2015-05-27 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-9000000000-4430d6a790eca4132aa4 | 2015-05-27 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-9000000000-607a755de038203a6b68 | 2015-05-27 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9000000000-63c9f623d8dc4b1e60a2 | 2015-05-27 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-9000000000-00ba25458eb6c0cc2940 | 2021-09-22 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-9000000000-00ba25458eb6c0cc2940 | 2021-09-22 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0002-9000000000-0d922cdfd7f6947230c0 | 2021-09-22 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-9000000000-452a5f79625d3401d495 | 2021-09-23 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-9000000000-452a5f79625d3401d495 | 2021-09-23 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9000000000-2758497e574a09010547 | 2021-09-23 | View Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-002f-9000000000-65d53ef91644a0bacd6c | 2014-09-20 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 300 MHz, CDCl3, experimental) | Not Available | 2014-09-20 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 50.18 MHz, CDCl3, experimental) | Not Available | 2014-09-23 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, CDCl3, experimental) | Not Available | 2016-10-22 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 100 MHz, H2O, predicted) | Not Available | 2022-08-22 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 100 MHz, H2O, predicted) | Not Available | 2022-08-22 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, H2O, predicted) | Not Available | 2022-08-22 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, H2O, predicted) | Not Available | 2022-08-22 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 200 MHz, H2O, predicted) | Not Available | 2022-08-22 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 200 MHz, H2O, predicted) | Not Available | 2022-08-22 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 300 MHz, H2O, predicted) | Not Available | 2022-08-22 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 300 MHz, H2O, predicted) | Not Available | 2022-08-22 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 400 MHz, H2O, predicted) | Not Available | 2022-08-22 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 400 MHz, H2O, predicted) | Not Available | 2022-08-22 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 500 MHz, H2O, predicted) | Not Available | 2022-08-22 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 500 MHz, H2O, predicted) | Not Available | 2022-08-22 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 600 MHz, H2O, predicted) | Not Available | 2022-08-22 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 600 MHz, H2O, predicted) | Not Available | 2022-08-22 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 700 MHz, H2O, predicted) | Not Available | 2022-08-22 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 700 MHz, H2O, predicted) | Not Available | 2022-08-22 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 800 MHz, H2O, predicted) | Not Available | 2022-08-22 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 800 MHz, H2O, predicted) | Not Available | 2022-08-22 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 900 MHz, H2O, predicted) | Not Available | 2022-08-22 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 900 MHz, H2O, predicted) | Not Available | 2022-08-22 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Acetaldehyde can form adducts with DNA, causing damage such as cross-links. (1) |
|---|
| Metabolism | In the liver, the enzyme acetaldehyde dehydrogenase converts acetaldehyde into the harmless compound acetic acid. (25) |
|---|
| Toxicity Values | LD50: 661 mg/kg (Oral, Rat) (21)
LD50: 212 mg/kg (Intravenous, Mouse) (21)
LD50: 3540 mg/kg (Dermal, Rabbit) (21)
LD50: 640 mg/kg (Subcutaneous, Rat) (22)
LD50: 96 mg/kg (Intratracheal, Hamster) (21)
LD50: 500 mg/kg (Intraperitoneal, Mouse) (26)
LC50: 1500 ppm over 4 hours (Inhalation, Mouse) (21) |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | 2B, possibly carcinogenic to humans. Acetaldehyde associated with consumption of alcoholic beverages is carcinogenic to humans (Group 1). (24) |
|---|
| Uses/Sources | Acetaldehyde occurs naturally in ripe fruit, coffee, and bread, and is produced by plants as part of their normal metabolism. It is popularly known as a chemical that causes hangovers, as it is produced from the breakdown of ethanol. In the chemical industry, acetaldehyde is used as an intermediate in the production of acetic acid, certain esters, and a number of other chemicals. it is also an air pollutant resulting from combustion, such as automotive exhaust and tobacco smoke. (25) |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Acetaldehyde is a probable carcinogen. (25) |
|---|
| Symptoms | Skin contact with acetaldehyde causes irritation. (25) |
|---|
| Treatment | Not Available |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB00990 |
|---|
| PubChem Compound ID | 177 |
|---|
| ChEMBL ID | CHEMBL170365 |
|---|
| ChemSpider ID | 172 |
|---|
| KEGG ID | C00084 |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | 100650 |
|---|
| ChEBI ID | 15343 |
|---|
| BioCyc ID | ACETALD |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Acetaldehyde |
|---|
| PDB ID | ACE |
|---|
| ACToR ID | 3 |
|---|
| Wikipedia Link | Acetaldehyde |
|---|
| References |
|---|
| Synthesis Reference | Wertheim, E. Laboratory preparation of acetaldehyde. Journal of the American Chemical Society (1922), 44 2658-9. |
|---|
| MSDS | Link |
|---|
| General References | - Brooks PJ, Theruvathu JA: DNA adducts from acetaldehyde: implications for alcohol-related carcinogenesis. Alcohol. 2005 Apr;35(3):187-93. [16054980 ]
- Nakamura K, Iwahashi K, Furukawa A, Ameno K, Kinoshita H, Ijiri I, Sekine Y, Suzuki K, Iwata Y, Minabe Y, Mori N: Acetaldehyde adducts in the brain of alcoholics. Arch Toxicol. 2003 Oct;77(10):591-3. Epub 2003 Sep 17. [14574447 ]
- Takeuchi M, Watai T, Sasaki N, Choei H, Iwaki M, Ashizawa T, Inagaki Y, Yamagishi S, Kikuchi S, Riederer P, Saito T, Bucala R, Kameda Y: Neurotoxicity of acetaldehyde-derived advanced glycation end products for cultured cortical neurons. J Neuropathol Exp Neurol. 2003 May;62(5):486-96. [12769188 ]
- Higuchi S, Matsushita S, Masaki T, Yokoyama A, Kimura M, Suzuki G, Mochizuki H: Influence of genetic variations of ethanol-metabolizing enzymes on phenotypes of alcohol-related disorders. Ann N Y Acad Sci. 2004 Oct;1025:472-80. [15542751 ]
- Oba T, Maeno Y, Ishida K: Differential contribution of clinical amounts of acetaldehyde to skeletal and cardiac muscle dysfunction in alcoholic myopathy. Curr Pharm Des. 2005;11(6):791-80. [15777233 ]
- Nishimura FT, Fukunaga T, Kajiura H, Umeno K, Takakura H, Ono T, Nishijo H: Effects of aldehyde dehydrogenase-2 genotype on cardiovascular and endocrine responses to alcohol in young Japanese subjects. Auton Neurosci. 2002 Nov 29;102(1-2):60-70. [12492137 ]
- Boyden TW, Silvert MA, Pamenter RW: Acetaldehyde acutely impairs canine testicular testosterone secretion. Eur J Pharmacol. 1981 Apr 9;70(4):571-6. [7195339 ]
- Theruvathu JA, Jaruga P, Nath RG, Dizdaroglu M, Brooks PJ: Polyamines stimulate the formation of mutagenic 1,N2-propanodeoxyguanosine adducts from acetaldehyde. Nucleic Acids Res. 2005 Jun 21;33(11):3513-20. Print 2005. [15972793 ]
- Burton A: Acetaldehyde links alcohol consumption to cancer. Lancet Oncol. 2005 Sep;6(9):643. [16161263 ]
- Hard ML, Iqbal U, Brien JF, Koren G: Binding of acetaldehyde to human and Guinea pig placentae in vitro. Placenta. 2003 Feb-Mar;24(2-3):149-54. [12566241 ]
- Forn-Frias C, Sanchis-Segura C: [The possible role of acetaldehyde in the brain damage caused by the chronic consumption of alcohol]. Rev Neurol. 2003 Sep 1-15;37(5):485-93. [14533100 ]
- Deitrich RA: Acetaldehyde: deja vu du jour. J Stud Alcohol. 2004 Sep;65(5):557-72. [15536764 ]
- Tyulina OV, Prokopieva VD, Boldyrev AA, Johnson P: Erythrocyte and plasma protein modification in alcoholism: a possible role of acetaldehyde. Biochim Biophys Acta. 2006 May;1762(5):558-63. Epub 2006 Apr 3. [16630710 ]
- Morozov IuE, Salomatin EM, Okhotin VE: [Brain acetaldehyde and ethanol: method of determination and diagnostic significance in ethanol poisoning]. Sud Med Ekspert. 2002 Mar-Apr;45(2):35-40. [12063798 ]
- Tyulina OV, Prokopieva VD, Dodd RD, Hawkins JR, Clay SW, Wilson DO, Boldyrev AA, Johnson P: In vitro effects of ethanol, acetaldehyde and fatty acid ethyl esters on human erythrocytes. Alcohol Alcohol. 2002 Mar-Apr;37(2):179-86. [11912075 ]
- Matsuse H, Shimoda T, Fukushima C, Mitsuta K, Kawano T, Tomari S, Saeki S, Kondoh Y, Machida I, Obase Y, Asai S, Kohno S: Screening for acetaldehyde dehydrogenase 2 genotype in alcohol-induced asthma by using the ethanol patch test. J Allergy Clin Immunol. 2001 Nov;108(5):715-9. [11692094 ]
- Yokoyama T, Saito K, Lwin H, Yoshiike N, Yamamoto A, Matsushita Y, Date C, Tanaka H: Epidemiological evidence that acetaldehyde plays a significant role in the development of decreased serum folate concentration and elevated mean corpuscular volume in alcohol drinkers. Alcohol Clin Exp Res. 2005 Apr;29(4):622-30. [15834228 ]
- Mascia MP, Maiya R, Borghese CM, Lobo IA, Hara K, Yamakura T, Gong DH, Beckstead MJ: Does acetaldehyde mediate ethanol action in the central nervous system? Alcohol Clin Exp Res. 2001 Nov;25(11):1570-5. [11707631 ]
- Takeuchi M, Saito T: Cytotoxicity of acetaldehyde-derived advanced glycation end-products (AA-AGE) in alcoholic-induced neuronal degeneration. Alcohol Clin Exp Res. 2005 Dec;29(12 Suppl):220S-4S. [16385226 ]
- Latvala J, Melkko J, Parkkila S, Jarvi K, Makkonen K, Niemela O: Assays for acetaldehyde-derived adducts in blood proteins based on antibodies against acetaldehyde/lipoprotein condensates. Alcohol Clin Exp Res. 2001 Nov;25(11):1648-53. [11707639 ]
- Lewis RJ (1996). Sax's Dangerous Properties of Industrial Materials. 9th ed. Volumes 1-3. New York, NY: Van Nostrand Reinhold.
- ITII (1988). Toxic and Hazardous Industrial Chemicals Safety Manual. Tokyo, Japan: The International Technical Information Institute.
- WHO (1995). Environmental Health Criteria 167: Acetaldehyde.
- International Agency for Research on Cancer (2014). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. [Link]
- Wikipedia. Acetaldehyde. Last Updated 17 July 2009. [Link]
- Drugs.com [Link]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | | Gene | Gene Symbol | Gene ID | Interaction | Chromosome | Details |
|---|
|
|---|
| Down-Regulated Genes | | Gene | Gene Symbol | Gene ID | Interaction | Chromosome | Details |
|---|
|
|---|