| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2009-07-30 17:59:13 UTC |
|---|
| Update Date | 2014-12-24 20:26:08 UTC |
|---|
| Accession Number | T3D3542 |
|---|
| Identification |
|---|
| Common Name | Valaciclovir |
|---|
| Class | Small Molecule |
|---|
| Description | Valaciclovir (INN) or valacyclovir (USAN) is an antiviral drug used in the management of herpes simplex and herpes zoster (shingles). It is a prodrug, being converted in vivo to aciclovir. It is marketed by GlaxoSmithKline under the trade name Valtrex or Zelitrex. [Wikipedia] |
|---|
| Compound Type | - Amide
- Amine
- Antiviral Agent
- Drug
- Ester
- Ether
- Metabolite
- Organic Compound
- Prodrug
- Synthetic Compound
|
|---|
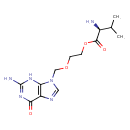
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | Bagovir | | Cycloval | | L-Valine ester with 9-((2-hydroxyethoxy)methyl)guanine | | L-Valine, 2-((2-amino-1,6-dihydro-6-oxo-9H-purin-9-yl)methoxy)ethyl ester | | Mitanga | | Ovalac | | Pervioral | | Revira | | Vacyless | | Vadiral | | Valaciclovirum | | Valacyclover Hydrochloric | | Valacyclovir | | Valdacir | | Valotix | | Valtrex | | Valvir | | Valztrex | | Viramixal | | Viropel | | Vociflon | | Zelitrex | | Zelivire |
|
|---|
| Chemical Formula | C13H20N6O4 |
|---|
| Average Molecular Mass | 324.336 g/mol |
|---|
| Monoisotopic Mass | 324.155 g/mol |
|---|
| CAS Registry Number | 124832-26-4 |
|---|
| IUPAC Name | 2-[(2-amino-6-oxo-6,9-dihydro-3H-purin-9-yl)methoxy]ethyl (2S)-2-amino-3-methylbutanoate |
|---|
| Traditional Name | valtrex |
|---|
| SMILES | [H][C@](N)(C(C)C)C(=O)OCCOCN1C=NC2=C1NC(=N)N=C2O |
|---|
| InChI Identifier | InChI=1S/C13H20N6O4/c1-7(2)8(14)12(21)23-4-3-22-6-19-5-16-9-10(19)17-13(15)18-11(9)20/h5,7-8H,3-4,6,14H2,1-2H3,(H3,15,17,18,20)/t8-/m0/s1 |
|---|
| InChI Key | InChIKey=HDOVUKNUBWVHOX-QMMMGPOBSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as alpha amino acid esters. These are ester derivatives of alpha amino acids. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Alpha amino acid esters |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alpha-amino acid ester
- Valine or derivatives
- 6-oxopurine
- Hypoxanthine
- Imidazopyrimidine
- Purine
- Aminopyrimidine
- Pyrimidone
- Fatty acid ester
- N-substituted imidazole
- Pyrimidine
- Fatty acyl
- Azole
- Heteroaromatic compound
- Vinylogous amide
- Imidazole
- Carboxylic acid ester
- Azacycle
- Organoheterocyclic compound
- Monocarboxylic acid or derivatives
- Organooxygen compound
- Primary amine
- Organic oxygen compound
- Organic nitrogen compound
- Hydrocarbon derivative
- Carbonyl group
- Organic oxide
- Organopnictogen compound
- Primary aliphatic amine
- Amine
- Organonitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | - Cytoplasm
- Extracellular
- Membrane
|
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | The blue, film-coated caplets are printed with edible white ink. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | 3.55e+00 g/L | | LogP | -0.3 |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00di-9210000000-51af1895b69904867582 | 2017-09-01 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-0udi-1910000000-3db14f76ba496cea0d81 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 35V, Positive | splash10-0udi-2900000000-a3e92ac9125245cf5513 | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 35V, Negative | splash10-016r-0910000000-01375a23cf811e488a21 | 2021-09-20 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-1913000000-e8d1da3daee2d7637783 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-2900000000-90825e6bac8b121f346e | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0zg0-2900000000-9ced6b2b253471425de0 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00xs-5913000000-05fdd28b28d4365d3f85 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-106s-5922000000-36d450a0ce5422cc73d6 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0pbc-5900000000-8eaf432e968b85e4cb9a | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0fb9-0926000000-779dfd0f2b063ec5e6c4 | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-0901000000-0d030c844b5ab6d9d46d | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000i-2900000000-7c25463514f877a34043 | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0359000000-0a84f61843d1db09c776 | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-2920000000-8736ab32172119625d46 | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0k9x-5900000000-21c409fb0971f216644d | 2021-10-11 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | 2021-09-25 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | After oral administration, valaciclovir hydrochloride is rapidly absorbed from the gastrointestinal tract. The absolute bioavailability of acyclovir after administration of valaciclovir is 54.5% ± 9.1%. |
|---|
| Mechanism of Toxicity | Valaciclovir is phosphorylated by viral thymidine kinase to acyclovir triphosphate (the active metabolite) which then inhibits herpes viral DNA replication by competitive inhibition of viral DNA polymerase, and by incorporation into and termination of the growing viral DNA chain. When used as a substrate for viral DNA polymerase, acyclovir triphosphate competitively inhibits dATP leading to the formation of 'faulty' DNA. This is where acyclovir triphosphate is incorporated into the DNA strand replacing many of the adenosine bases. This results in the prevention of DNA synthesis, as phosphodiester bridges can longer to be built, destabilizing the strand. |
|---|
| Metabolism | Valaciclovir is rapidly and almost entirely (~99%) converted to the active compound, acyclovir, and L-valine by first-pass intestinal and hepatic metabolism by enzymatic hydrolysis. Neither valaciclovir nor acyclovir is metabolized by cytochrome P450 enzymes. Acyclovir is converted to a small extent to inactive metabolites by aldehyde oxidase and by alcohol and aldehyde dehydrogenase. Plasma concentrations of unconverted valacyclovir are low and transient, generally becoming non-quantifiable by 3 hours after administration. Peak plasma valacyclovir concentrations are generally less than 0.5 mcg/mL at all doses. After single-dose administration of 1 gram of VALTREX, average plasma valacyclovir concentrations observed were 0.5, 0.4, and 0.8 mcg/mL in patients with hepatic dysfunction, renal insufficiency, and in healthy volunteers who received concomitant cimetidine and probenecid, respectively.
Route of Elimination: Acyclovir accounted for 89% of the radioactivity excreted in the urine.
Half Life: 2.5-3.3 hours |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | For the treatment or suppression of cold sores (herpes labialis), herpes zoster (shingles), genital herpes in immunocompetent individuals, and recurrent genital herpes in HIV-infected individuals.
Cold Sores (Herpes Labialis): VALTREX is indicated for treatment of cold sores (herpes labialis). The efficacy of VALTREX initiated after the development of clinical signs of a cold sore (e.g., papule, vesicle, or ulcer) has not been established.
Genital Herpes: Initial Episode: VALTREX is indicated for treatment of the initial episode of genital herpes in immunocompetent adults. The efficacy of treatment with VALTREX when initiated more than 72 hours after the onset of signs and symptoms has not been established. |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | General: Facial edema, hypertension, tachycardia.
Allergic:Acute hypersensitivity reactions including anaphylaxis, angioedema, dyspnea, pruritus, rash, and urticaria.
CNS Symptoms: Aggressive behavior; agitation; ataxia; coma; confusion; decreased consciousness; dysarthria; encephalopathy; mania; and psychosis, including auditory and visual hallucinations, seizures, tremors.
Eye: Visual abnormalities.
Gastrointestinal: Diarrhea.
Hepatobiliary Tract and Pancreas: Liver enzyme abnormalities, hepatitis.
Renal: Renal failure, renal pain (may be associated with renal failure).
Hematologic: Thrombocytopenia, aplastic anemia, leukocytoclastic vasculitis, TTP/HUS.
Skin: Erythema multiforme, rashes including photosensitivity, alopecia. |
|---|
| Symptoms | Adverse effects of overexposure might include headache and nausea. |
|---|
| Treatment | In the event of acute renal failure and anuria, the patient may benefit from hemodialysis until renal function is restored. (5) |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB00577 |
|---|
| HMDB ID | HMDB14716 |
|---|
| PubChem Compound ID | 60773 |
|---|
| ChEMBL ID | CHEMBL1349 |
|---|
| ChemSpider ID | 54770 |
|---|
| KEGG ID | C07184 |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | 35854 |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Valaciclovir |
|---|
| PDB ID | TXC |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Valaciclovir |
|---|
| References |
|---|
| Synthesis Reference | Marina Etinger, “Synthesis and purification of valacyclovir.” U.S. Patent US20030153757, issued August 14, 2003. |
|---|
| MSDS | Link |
|---|
| General References | - O'Brien JJ, Campoli-Richards DM: Acyclovir. An updated review of its antiviral activity, pharmacokinetic properties and therapeutic efficacy. Drugs. 1989 Mar;37(3):233-309. [2653790 ]
- Umapathy NS, Ganapathy V, Ganapathy ME: Transport of amino acid esters and the amino-acid-based prodrug valganciclovir by the amino acid transporter ATB(0,+). Pharm Res. 2004 Jul;21(7):1303-10. [15290873 ]
- Drugs.com [Link]
- RxList: The Internet Drug Index (2009). [Link]
- RxList: The Internet Drug Index (2009). [Link]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|