| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2009-12-17 01:41:03 UTC |
|---|
| Update Date | 2018-03-21 17:46:25 UTC |
|---|
| Accession Number | T3D3660 |
|---|
| Identification |
|---|
| Common Name | Glycolic acid |
|---|
| Class | Small Molecule |
|---|
| Description | Glycolic acid (or hydroxyacetic acid) is the smallest alpha-hydroxy acid (AHA). This colourless, odourless, and hygroscopic crystalline solid is highly soluble in water. Due to its excellent capability to penetrate skin, glycolic acid is often used in skin care products, most often as a chemical peel. It may reduce wrinkles, acne scarring, and hyperpigmentation and improve many other skin conditions, including actinic keratosis, hyperkeratosis, and seborrheic keratosis. Once applied, glycolic acid reacts with the upper layer of the epidermis, weakening the binding properties of the lipids that hold the dead skin cells together. This allows the outer skin to dissolve, revealing the underlying skin. It is thought that this is due to the reduction of calcium ion concentrations in the epidermis and the removal of calcium ions from cell adhesions, leading to desquamation. Glycolic acid is a known inhibitor of tyrosinase. This can suppress melanin formation and lead to a lightening of skin colour. Acute doses of glycolic acid on skin or eyes leads to local effects that are typical of a strong acid (e.g. dermal and eye irritation). Glycolate is a nephrotoxin if consumed orally. A nephrotoxin is a compound that causes damage to the kidney and kidney tissues. Glycolic acid's renal toxicity is due to its metabolism to oxalic acid. Glycolic and oxalic acid, along with excess lactic acid, are responsible for the anion gap metabolic acidosis. Oxalic acid readily precipitates with calcium to form insoluble calcium oxalate crystals. Renal tissue injury is caused by widespread deposition of oxalate crystals and the toxic effects of glycolic acid. Glycolic acid does exhibit some inhalation toxicity and can cause respiratory, thymus, and liver damage if present in very high levels over long periods of time. |
|---|
| Compound Type | - Cosmetic Toxin
- Food Toxin
- Household Toxin
- Industrial/Workplace Toxin
- Keratolytic Agent
- Metabolite
- Organic Compound
- Synthetic Compound
|
|---|
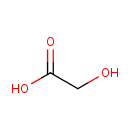
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | 2-Hydroxyacetate | | 2-Hydroxyacetic acid | | a-Hydroxyacetate | | a-Hydroxyacetic acid | | alpha-Hydroxyacetate | | alpha-Hydroxyacetic acid | | Glycocide | | Glycolate | | Glycollate | | Glycollic acid | | GlyPure | | GlyPure 70 | | Hydroxyacetate | | Hydroxyacetic acid | | Hydroxyethanoate | | Hydroxyethanoic acid | | Sodium glycolate |
|
|---|
| Chemical Formula | C2H4O3 |
|---|
| Average Molecular Mass | 76.051 g/mol |
|---|
| Monoisotopic Mass | 76.016 g/mol |
|---|
| CAS Registry Number | 79-14-1 |
|---|
| IUPAC Name | 2-hydroxyacetic acid |
|---|
| Traditional Name | glycolic acid |
|---|
| SMILES | OCC(O)=O |
|---|
| InChI Identifier | InChI=1S/C2H4O3/c3-1-2(4)5/h3H,1H2,(H,4,5) |
|---|
| InChI Key | InChIKey=AEMRFAOFKBGASW-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as alpha hydroxy acids and derivatives. These are organic compounds containing a carboxylic acid substituted with a hydroxyl group on the adjacent carbon. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Hydroxy acids and derivatives |
|---|
| Sub Class | Alpha hydroxy acids and derivatives |
|---|
| Direct Parent | Alpha hydroxy acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alpha-hydroxy acid
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Primary alcohol
- Organooxygen compound
- Carbonyl group
- Alcohol
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Endogenous |
|---|
| Cellular Locations | - Cytoplasm
- Extracellular
- Mitochondria
- Peroxisome
|
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | - Bladder
- Fibroblasts
- Liver
- Skin
- Stratum Corneum
|
|---|
| Pathways | | Name | SMPDB Link | KEGG Link |
|---|
| Primary Hyperoxaluria Type I | SMP00352 | Not Available |
|
|---|
| Applications | |
|---|
| Biological Roles | |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | 75-80°C | | Boiling Point | Not Available | | Solubility | 1000 mg/mL at 25°C | | LogP | -1.11 |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (Non-derivatized) | splash10-0002-0900000000-ed8b8e4a9e2556ea02e2 | 2014-06-16 | View Spectrum | | GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (2 TMS) | splash10-00dj-9600000000-8bafc88c7bf4e90fb5e8 | 2014-06-16 | View Spectrum | | GC-MS | GC-MS Spectrum - GC-MS (2 TMS) | splash10-003r-2910000000-bd50bf5bab6f5327eaf4 | 2014-06-16 | View Spectrum | | GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-001i-9000000000-cadf899be6b15d008330 | 2017-09-12 | View Spectrum | | GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-001i-9000000000-e66ed28d8419895e0fb4 | 2017-09-12 | View Spectrum | | GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0002-0900000000-7f84fac3284d17fa3ba6 | 2017-09-12 | View Spectrum | | GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-0002-0900000000-ed8b8e4a9e2556ea02e2 | 2017-09-12 | View Spectrum | | GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-00dj-9600000000-8bafc88c7bf4e90fb5e8 | 2017-09-12 | View Spectrum | | GC-MS | GC-MS Spectrum - GC-MS (Non-derivatized) | splash10-003r-2910000000-bd50bf5bab6f5327eaf4 | 2017-09-12 | View Spectrum | | GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-0002-0900000000-d724c85a3b30e3c2e4bc | 2017-09-12 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0a7l-9000000000-1e9466549305eb20257b | 2016-09-22 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-05i9-9520000000-5f0019fe63eb6e692109 | 2017-10-06 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | 2021-11-05 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | 2021-11-05 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | 2021-11-05 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | 2021-11-05 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_1) - 70eV, Positive | Not Available | 2021-11-05 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Negative (Annotated) | splash10-004i-9000000000-e942bdae1d60e5f5d649 | 2012-07-24 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Negative (Annotated) | splash10-00di-9000000000-f225de2de3540c3f50a4 | 2012-07-24 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Negative (Annotated) | splash10-00di-9000000000-7de217d97b44f53aad82 | 2012-07-24 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 10V, Negative | splash10-00di-9000000000-88af2b259f82cd1d8938 | 2012-08-31 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 20V, Negative | splash10-004i-9000000000-c968a24f0640b154325b | 2012-08-31 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 30V, Negative | splash10-0059-9000000000-1dfacf30bf94ce3bf8bb | 2012-08-31 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , negative | splash10-00di-9000000000-88af2b259f82cd1d8938 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , negative | splash10-004i-9000000000-c968a24f0640b154325b | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , negative | splash10-0059-9000000000-1dfacf30bf94ce3bf8bb | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - QqQ 1V, positive | splash10-004i-9000000000-fa715ee3ce9abbc94edb | 2020-07-22 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - QqQ 2V, positive | splash10-004i-9000000000-ace3c5f526d28fd24de9 | 2020-07-22 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - QqQ 3V, positive | splash10-004i-9000000000-ff7f922c2460adb6a10d | 2020-07-22 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - QqQ 4V, positive | splash10-004i-9000000000-1d63aaaf9cbc6d3bb3b4 | 2020-07-22 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - QqQ 5V, positive | splash10-004j-9000000000-e9c74e7df728e016450c | 2020-07-22 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - QqQ 6V, positive | splash10-002b-9000000000-d0963403f3a8ff9e576d | 2020-07-22 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - QqQ 7V, positive | splash10-002b-9000000000-9e1d6b2e2b889232d610 | 2020-07-22 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - QqQ 8V, positive | splash10-0002-9000000000-e9824f68b2176db90d33 | 2020-07-22 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - QqQ 9V, positive | splash10-000t-9000000000-02329982bc7150d972bf | 2020-07-22 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - QqQ 10V, positive | splash10-000t-9000000000-216d8ece56b0a44e5289 | 2020-07-22 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-9000000000-d961c3c14ec415e3141e | 2015-04-24 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a6r-9000000000-67f73be970ba9f885c4a | 2015-04-24 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-9000000000-f2ccf0b88e0ad65ed4c6 | 2015-04-24 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-9000000000-7445713a5fe347bbc8b8 | 2015-04-25 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-9000000000-26e13242443efc1aa846 | 2015-04-25 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-9000000000-6ba976b949118cd0a86a | 2015-04-25 | View Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-001i-9000000000-2885890e3bb8c015742f | 2014-09-20 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 500 MHz, H2O, experimental) | Not Available | 2012-12-04 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, experimental) | Not Available | 2014-09-20 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 25.16 MHz, D2O, experimental) | Not Available | 2014-09-23 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, D2O, experimental) | Not Available | 2016-10-22 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, D2O, experimental) | Not Available | 2016-10-22 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, H2O, experimental) | Not Available | 2012-12-04 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Oral (30) ; dermal (30) |
|---|
| Mechanism of Toxicity | Glycolic acid's toxicity is due to its metabolism to oxalic acid. Glycolic and oxalic acid, along with excess lactic acid, are responsible for the anion gap metabolic acidosis. Oxalic acid readily precipitates with calcium to form insoluble calcium oxalate crystals. Tissue injury is caused by widespread deposition of oxalate crystals and the toxic effects of glycolic acid. (1, 2) |
|---|
| Metabolism | The main path of the degradation of glycolic acid is to glyoxylic acid. This reaction is mediated by lactic dehydrogenase or glycolic acid oxidase. Once glyoxylic acid is formed, it is apparently degraded very rapidly to a variety of products, a few of which have been observed. Its breakdown to 2-hydroxy-3-oxoadipate it is thought, is mediated by thiamine pyrophosphate in the presence of magnesium ions. The formation of glycine involves pyridoxal phosphate and glyoxylate transaminase, whereas the formation of carbon dioxide and water via formic acid apparently involves coenzyme A (CoA) and flavin mononucleotides. (25) |
|---|
| Toxicity Values | LD50: 1950 mg/kg (Oral, Rat) (3)
LD50: 1000 mg/kg (Intravenous, Cat) (4)
LC50: 7.7-14 mg/L over 4 hours (Inhalation, Rat) (4) |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | Due to its excellent capability to penetrate skin, glycolic acid finds applications in skin care products, most often as a chemical peel. It may reduce wrinkles, acne scarring, hyperpigmentation and improve many other skin conditions, including actinic keratosis, hyperkeratosis, and seborrheic keratosis. Glycolic acid is also a useful intermediate for organic synthesis and finds employment in the textile industry as a dyeing and tanning agent, in food processing as a flavoring agent and as a preservative. Glycolic acid is often included into emulsion polymers, solvents and additives for ink and paint in order to improve flow properties and impart gloss. (30) |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Glycolic acid metabolizes to oxalic acid, which reacts with calcium and forms calcium oxalate crystals in the kidney. This can cause kidney injury, leading to acute kidney failure. (31)
Chronically high levels of glycolic acid are associated with the inborn error of metabolism known as Type I primary hyperoxaluria. Oxalate stones in primary hyperoxaluria tend to be severe, resulting in relatively early kidney damage (before age 20), which impairs the excretion of oxalate leading to a further acceleration in accumulation of oxalate in the body. After the development of renal failure patients may develop oxalate deposits in the bones, joints and bone marrow. Severe cases may develop haematological problems such as anaemia and thrombocytopaenia. The deposition of oxalate in the body is sometimes called "oxalosis" to be distinguished from "oxaluria" which refers to oxalate in the urine. |
|---|
| Symptoms | Glycolic acid is a strong irritant. Accumulation of glycolic acid and its metabolite, oxalic acid, causes tachycardia, hypertension, hyperventilation, and metabolic acidosis. (31, 30) |
|---|
| Treatment | Chronic Exposure: In some patients with primary hyperoxaluria type 1, pyridoxine treatment (vitamin B6) may decrease oxalate excretion and prevent kidney stone formation.
Acute Exposure: EYES: irrigate opened eyes for several minutes under running water. INGESTION: do not induce vomiting. Rinse mouth with water (never give anything by mouth to an unconscious person). Seek immediate medical advice. |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB03085 |
|---|
| HMDB ID | HMDB00115 |
|---|
| PubChem Compound ID | 757 |
|---|
| ChEMBL ID | CHEMBL252557 |
|---|
| ChemSpider ID | 737 |
|---|
| KEGG ID | C00160 |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | 259900 , 260000 |
|---|
| ChEBI ID | 29805 |
|---|
| BioCyc ID | GLYCOLLATE |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Not Available |
|---|
| PDB ID | GOA |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Glycolic_acid |
|---|
| References |
|---|
| Synthesis Reference | David Y. Tang, Arthur M. Foster, “(3-Trifluoromethylphenyl)-alpha-hydroxyacetic acid and process for preparation.” U.S. Patent US4296244, issued January, 1977. |
|---|
| MSDS | Link |
|---|
| General References | - Yamamoto N, Naraparaju VR: Vitamin D3-binding protein as a precursor for macrophage activating factor in the inflammation-primed macrophage activation cascade in rats. Cell Immunol. 1996 Jun 15;170(2):161-7. [8660814 ]
- Yamamoto N, Naraparaju VR: Role of vitamin D3-binding protein in activation of mouse macrophages. J Immunol. 1996 Aug 15;157(4):1744-9. [8759764 ]
- de Leon J, Tracy J, McCann E, McGrory A, Diaz FJ: Schizophrenia and tobacco smoking: a replication study in another US psychiatric hospital. Schizophr Res. 2002 Jul 1;56(1-2):55-65. [12084420 ]
- Haskell CF, Kennedy DO, Wesnes KA, Milne AL, Scholey AB: A double-blind, placebo-controlled, multi-dose evaluation of the acute behavioural effects of guarana in humans. J Psychopharmacol. 2007 Jan;21(1):65-70. Epub 2006 Mar 13. [16533867 ]
- Horikoshi T, Matsumoto M, Usuki A, Igarashi S, Hikima R, Uchiwa H, Hayashi S, Brysk MM, Ichihashi M, Funasaka Y: Effects of glycolic acid on desquamation-regulating proteinases in human stratum corneum. Exp Dermatol. 2005 Jan;14(1):34-40. [15660917 ]
- Shoemaker JD, Elliott WH: Automated screening of urine samples for carbohydrates, organic and amino acids after treatment with urease. J Chromatogr. 1991 Jan 2;562(1-2):125-38. [2026685 ]
- DiNardo JC, Grove GL, Moy LS: Clinical and histological effects of glycolic acid at different concentrations and pH levels. Dermatol Surg. 1996 May;22(5):421-4. [8634803 ]
- Marangella M, Petrarulo M, Bianco O, Vitale C, Finocchiaro P, Linari F: Glycolate determination detects type I primary hyperoxaluria in dialysis patients. Kidney Int. 1991 Jan;39(1):149-54. [2002628 ]
- Tsiafoulis CG, Prodromidis MI, Karayannis MI: Development of amperometric biosensors for the determination of glycolic acid in real samples. Anal Chem. 2002 Jan 1;74(1):132-9. [11795781 ]
- Porter WH, Rutter PW, Yao HH: Simultaneous determination of ethylene glycol and glycolic acid in serum by gas chromatography-mass spectrometry. J Anal Toxicol. 1999 Nov-Dec;23(7):591-7. [10595845 ]
- Jacobsen D, Hewlett TP, Webb R, Brown ST, Ordinario AT, McMartin KE: Ethylene glycol intoxication: evaluation of kinetics and crystalluria. Am J Med. 1988 Jan;84(1):145-52. [3337119 ]
- Guneral F, Bachmann C: Age-related reference values for urinary organic acids in a healthy Turkish pediatric population. Clin Chem. 1994 Jun;40(6):862-6. [8087979 ]
- Bernstein EF, Lee J, Brown DB, Yu R, Van Scott E: Glycolic acid treatment increases type I collagen mRNA and hyaluronic acid content of human skin. Dermatol Surg. 2001 May;27(5):429-33. [11359487 ]
- Leumann EP, Dietl A, Matasovic A: Urinary oxalate and glycolate excretion in healthy infants and children. Pediatr Nephrol. 1990 Sep;4(5):493-7. [2242313 ]
- Booth ED, Dofferhoff O, Boogaard PJ, Watson WP: Comparison of the metabolism of ethylene glycol and glycolic acid in vitro by precision-cut tissue slices from female rat, rabbit and human liver. Xenobiotica. 2004 Jan;34(1):31-48. [14742135 ]
- Mahul P, Molliex S, Auboyer C, Levigne F, Jospe R, Dumont A, Gilloz A: [Neurotoxic role of glycocolle and derivatives in transurethral resection of the prostate]. Ann Fr Anesth Reanim. 1993;12(5):512-4. [8311360 ]
- Hoffmann GF, Meier-Augenstein W, Stockler S, Surtees R, Rating D, Nyhan WL: Physiology and pathophysiology of organic acids in cerebrospinal fluid. J Inherit Metab Dis. 1993;16(4):648-69. [8412012 ]
- Marangella M, Petrarulo M, Vitale C, Cosseddu D, Linari F: Plasma and urine glycolate assays for differentiating the hyperoxaluria syndromes. J Urol. 1992 Sep;148(3 Pt 2):986-9. [1507356 ]
- Effendy I, Kwangsukstith C, Lee JY, Maibach HI: Functional changes in human stratum corneum induced by topical glycolic acid: comparison with all-trans retinoic acid. Acta Derm Venereol. 1995 Nov;75(6):455-8. [8651024 ]
- Pien K, van Vlem B, van Coster R, Dacremont G, Piette M: An inherited metabolic disorder presenting as ethylene glycol intoxication in a young adult. Am J Forensic Med Pathol. 2002 Mar;23(1):96-100. [11953504 ]
- Dietzen DJ, Wilhite TR, Kenagy DN, Milliner DS, Smith CH, Landt M: Extraction of glyceric and glycolic acids from urine with tetrahydrofuran: utility in detection of primary hyperoxaluria. Clin Chem. 1997 Aug;43(8 Pt 1):1315-20. [9267307 ]
- Newman N, Newman A, Moy LS, Babapour R, Harris AG, Moy RL: Clinical improvement of photoaged skin with 50% glycolic acid. A double-blind vehicle-controlled study. Dermatol Surg. 1996 May;22(5):455-60. [8634809 ]
- Roe FJ: Perspectives in carbohydrate toxicology with special reference to carcinogenicity. Swed Dent J. 1984;8(3):99-111. [6592775 ]
- Porter WH, Rutter PW, Bush BA, Pappas AA, Dunnington JE: Ethylene glycol toxicity: the role of serum glycolic acid in hemodialysis. J Toxicol Clin Toxicol. 2001;39(6):607-15. [11762669 ]
- Bingham, E, Cohrssen, B, and Powell, CH (2001). Patty's Toxicology Volumes 1-9. 5th ed. New York, N.Y: John Wiley & Sons.
- Olson, KR (ed) (1999). Poisoning & Drug Overdose. 3rd edition. New York, NY: Lange Medical Books/McGraw-Hill.
- Gilman AG, Goodman LS, and Gilman A (eds) (1980). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 6th ed. New York, NY: Macmillan Publishing Co., Inc.
- Sax NI (1984). Dangerous Properties of Industrial Materials. 6th ed. New York, NY: Van Nostrand Reinhold.
- European Chemicals Bureau (2000). IUCLID Dataset, Glycollic acid (79-14-1).
- Wikipedia. Glycolic acid. Last Updated 27 October 2009, [Link]
- Wikidoc. Ethylene glycol. Last Updated 11 June 2009. [Link]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|