| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2013-04-25 07:56:55 UTC |
|---|
| Update Date | 2014-12-24 20:26:34 UTC |
|---|
| Accession Number | T3D3935 |
|---|
| Identification |
|---|
| Common Name | Tralkoxydim |
|---|
| Class | Small Molecule |
|---|
| Description | Tralkoxydim is a cyclohexanedione herbicide that is applied to actively growing weeds in wheat and barley to control wild oats, green foxtail, yellow foxtail, annual ryegrass (Italian) and persian darnel. |
|---|
| Compound Type | - Ester
- Ether
- Herbicide
- Organic Compound
- Pesticide
- Synthetic Compound
|
|---|
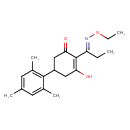
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | 2-((1E)-2-ethoxy-1-ethyl-2-azavinyl)-3-hydroxy-5-(2,4,6-trimethylphenyl)cyclohex-2-en-1-one | | 2-(1-(Ethoxyimino)propyl)-3-hydroxy-5-(2,4,6-trimethylphenyl)-2-cyclohexen-1-one | | 2-(1-Ethoxyimino-propyl)-3-hydroxy-5-(2,4,6-trimethyl-phenyl)-cyclohex-2-enone | | 2-(N-ethoxypropanimidoyl)-3-hydroxy-5-(2,4,6-trimethylphenyl)cyclohex-2-en-1-one | | 2-(N-ethoxypropanimidoyl)-3-hydroxy-5-mesitylcyclohex-2-en-1-one | | 2-[(1E)-N-ethoxypropanimidoyl]-3-hydroxy-5-(2,4,6-trimethylphenyl)cyclohex-2-en-1-one | | 2-[(1E)-N-Ethoxypropanimidoyl]-3-hydroxy-5-mesityl-2-cyclohexen-1-one | | 2-[(1E)-N-Ethoxypropanimidoyl]-3-hydroxy-5-mesitylcyclohex-2-en-1-one | | 2-[1-(ethoxyimino)propyl]-3-hydroxy-5-(2,4,6-trimethylphenyl)cyclohex-2-enone | | 2-{1-[(E)-Ethoxyimino]-propyl}-3-hydroxy-5-(2,4,6-trimethyl-phenyl)-cyclohex-2-enone | | Achieve | | Grasp | | Splendor | | Tralkoxidym |
|
|---|
| Chemical Formula | C20H27NO3 |
|---|
| Average Molecular Mass | 329.433 g/mol |
|---|
| Monoisotopic Mass | 329.199 g/mol |
|---|
| CAS Registry Number | 87820-88-0 |
|---|
| IUPAC Name | 2-[(1E)-1-(ethoxyimino)propyl]-3-hydroxy-5-(2,4,6-trimethylphenyl)cyclohex-2-en-1-one |
|---|
| Traditional Name | tralkoxydim |
|---|
| SMILES | CCON=C(CC)C1=C(O)CC(CC1=O)C1=C(C)C=C(C)C=C1C |
|---|
| InChI Identifier | InChI=1S/C20H27NO3/c1-6-16(21-24-7-2)20-17(22)10-15(11-18(20)23)19-13(4)8-12(3)9-14(19)5/h8-9,15,22H,6-7,10-11H2,1-5H3/b21-16+ |
|---|
| InChI Key | InChIKey=DQFPEYARZIQXRM-LTGZKZEYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as cyclohexenones. Cyclohexenones are compounds containing a cylohexenone moiety, which is a six-membered aliphatic ring that carries a ketone and has one endocyclic double bond. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbonyl compounds |
|---|
| Direct Parent | Cyclohexenones |
|---|
| Alternative Parents | |
|---|
| Substituents | - Cyclohexenone
- Benzenoid
- Monocyclic benzene moiety
- Vinylogous acid
- Oxime ether
- Enol
- Organic nitrogen compound
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Organonitrogen compound
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available | | LogP | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0149000000-a42e0351a7c63642063b | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0gc4-5594000000-0447c07e9184e2de9fc5 | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0hmk-8900000000-79943559a6af83ea52f9 | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0029000000-527d9b7b54a99326c1d5 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-003r-2091000000-2542af5511f0c750ffda | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052g-9230000000-2a72429be7cb41d48c98 | 2016-08-03 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Tralkoxydim is structurally a cyclohexanedione, but because it does not appear to produce a toxic metabolite produced by other substances, the EPA has not assumed that tralkoxydim has a common mechanism of toxicity with other substances (1). |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | This is a man-made compound that is used as a pesticide. |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| PubChem Compound ID | Not Available |
|---|
| ChEMBL ID | CHEMBL60556 |
|---|
| ChemSpider ID | 10469196 |
|---|
| KEGG ID | C18769 |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | T3D3935.pdf |
|---|
| General References | - Tralkoxydim; Notice of Filing a Pesticide Petition to Establish a Tolerance for a Certain Pesticide Chemical in or on Food. A Notice by the Environmental Protection Agency on 03/21/2003. [Link]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|