| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2014-08-29 04:49:32 UTC |
|---|
| Update Date | 2014-12-24 20:26:36 UTC |
|---|
| Accession Number | T3D4030 |
|---|
| Identification |
|---|
| Common Name | Dinophysistoxin 1 |
|---|
| Class | Small Molecule |
|---|
| Description | Dinophysistoxin 1 is found in mollusks. Dinophysistoxin 1 is a metabolite of Dinophysis fortii. Dinophysistoxin 1 is found in scallops and mussels. Component toxin in diarrhetic shellfish poisoning. Dinophysistoxin 1 belongs to the family of Okadaic Acids and Derivatives. These are heat-stable polyether and lipophilic compounds that accumulate in the fatty tissue of shellfish. |
|---|
| Compound Type | - Animal Toxin
- Ether
- Food Toxin
- Marine Toxin
- Metabolite
- Natural Compound
- Organic Compound
|
|---|
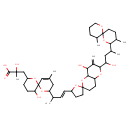
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | 35-Methyl-Okadaic acid | | 35-Methylokadaic acid | | 9,10-Deepithio-9,10-didehydro-35-methyl-Acanthifolicin | | Dinophysistoxin-1 | | DTX1 |
|
|---|
| Chemical Formula | C45H70O13 |
|---|
| Average Molecular Mass | 819.030 g/mol |
|---|
| Monoisotopic Mass | 818.482 g/mol |
|---|
| CAS Registry Number | 81720-10-7 |
|---|
| IUPAC Name | 3-{8-[(3E)-4-[6'-(3-{3,11-dimethyl-1,7-dioxaspiro[5.5]undecan-2-yl}-1-hydroxybutyl)-8'-hydroxy-7'-methylidene-hexahydro-3'H-spiro[oxolane-2,2'-pyrano[3,2-b]pyran]-5-yl]but-3-en-2-yl]-5-hydroxy-10-methyl-1,7-dioxaspiro[5.5]undec-10-en-2-yl}-2-hydroxy-2-methylpropanoic acid |
|---|
| Traditional Name | 3-{8-[(3E)-4-[6'-(3-{3,11-dimethyl-1,7-dioxaspiro[5.5]undecan-2-yl}-1-hydroxybutyl)-8'-hydroxy-7'-methylidene-hexahydrospiro[oxolane-2,2'-pyrano[3,2-b]pyran]-5-yl]but-3-en-2-yl]-5-hydroxy-10-methyl-1,7-dioxaspiro[5.5]undec-10-en-2-yl}-2-hydroxy-2-methylpropanoic acid |
|---|
| SMILES | [H]\C(C(C)C1CC(C)=CC2(OC(CC(C)(O)C(O)=O)CCC2O)O1)=C(\[H])C1CCC2(CCC3OC(C(O)CC(C)C4OC5(CCC4C)OCCCC5C)C(=C)C(O)C3O2)O1 |
|---|
| InChI Identifier | InChI=1/C45H70O13/c1-25-21-35(56-45(23-25)36(47)13-12-32(55-45)24-42(7,51)41(49)50)26(2)10-11-31-15-17-43(54-31)18-16-34-40(57-43)37(48)30(6)39(53-34)33(46)22-28(4)38-27(3)14-19-44(58-38)29(5)9-8-20-52-44/h10-11,23,26-29,31-40,46-48,51H,6,8-9,12-22,24H2,1-5,7H3,(H,49,50)/b11-10+ |
|---|
| InChI Key | InChIKey=CLBIEZBAENPDFY-ZHACJKMWNA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as ketals. These are acetals derived from ketones by replacement of the oxo group by two hydrocarbyloxy groups R2C(OR)2 ( R not Hydrogen ). This term, once abandoned, has been reinstated as a subclass of acetals. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Ethers |
|---|
| Direct Parent | Ketals |
|---|
| Alternative Parents | |
|---|
| Substituents | - Ketal
- Alpha-hydroxy acid
- Hydroxy acid
- Oxane
- Pyran
- Tertiary alcohol
- Tetrahydrofuran
- Secondary alcohol
- Organoheterocyclic compound
- Oxacycle
- Monocarboxylic acid or derivatives
- Dialkyl ether
- Carboxylic acid
- Carboxylic acid derivative
- Hydrocarbon derivative
- Carbonyl group
- Organic oxide
- Alcohol
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | 134°C | | Boiling Point | Not Available | | Solubility | Not Available | | LogP | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | 2021-10-19 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | 2021-10-19 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_3) - 70eV, Positive | Not Available | 2021-10-19 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_4) - 70eV, Positive | Not Available | 2021-10-19 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_5) - 70eV, Positive | Not Available | 2021-10-19 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0uy3-8403014970-3900c8eff5e7d68473e4 | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0006-9102056420-f8b6f3bd4a7ce9858ed6 | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05to-9311041200-93c2ee6c4d0dc6960f63 | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-9302110210-8b03578ad1101dc635a1 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-9218000500-6c987843e2b608dcd74a | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-046r-3936100100-0bdd11665939edd796ce | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0uxr-0000000290-b02747f80efb3804727d | 2021-09-25 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0v00-1421212190-77c7a82d2a9123cfa456 | 2021-09-25 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014i-8155974060-7c6f0b7a7dcb2285d2a3 | 2021-09-25 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0000000390-5e7e234b5a4144768d0d | 2021-09-25 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-2000010790-74dab7af892ec43f8adc | 2021-09-25 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0ar1-2394501450-03a03c3e03f7a68fffb7 | 2021-09-25 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Ingestion |
|---|
| Mechanism of Toxicity | Dinophysistoxin 1 (DTX1) is the analogue of okadaic acid causing diarrheic shellfish poisoning (DSP) in humans shortly after the ingestion of contaminated seafood. DTX1 is able to disrupt the integrity of Caco-2 monolayer cells at concentrations above 50 nM. In addition, confocal microscopy imaging confirmed that the tight-junction protein, occludin, was affected by DTX1. Permeability assays revealed that only DTX1 was able to significantly cross the intestinal epithelium at concentrations above 100 nM. The mechanism of action of this group of toxins is mainly the potent inhibition of serine/threonine protein phosphatases 1 (PP1) and 2A (PP2A). DTX1-treated Caco-2 monolayers could damage tight-junctions among cells. Tight-junctions form the physical barrier to the diffusion of substances through the paracellular space. |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | Dinophysistoxin 1 is found in mollusks. Dinophysistoxin 1 is a metabolite of Dinophysis fortii. Dinophysistoxin 1 is found in scallops and mussels. Component toxin in diarrhetic shellfish poisoning. Dinophysistoxin 1 belongs to the family of Okadaic Acids and Derivatives. |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB30442 |
|---|
| PubChem Compound ID | 6437058 |
|---|
| ChEMBL ID | Not Available |
|---|
| ChemSpider ID | 4941648 |
|---|
| KEGG ID | C16870 |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | - Yannai, Shmuel. (2004) Dictionary of food compounds with CD-ROM: Additives, flavors, and ingredients. Boca Raton: Chapman & Hall/CRC.
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|