| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2014-08-29 05:03:08 UTC |
|---|
| Update Date | 2014-12-24 20:26:37 UTC |
|---|
| Accession Number | T3D4074 |
|---|
| Identification |
|---|
| Common Name | Coriamyrtin |
|---|
| Class | Small Molecule |
|---|
| Description | The leaves and fruits of Coriaria myrtifolia contain coriamyrtin, a typical convulsant substance. |
|---|
| Compound Type | - Ester
- Ether
- Natural Compound
- Organic Compound
- Plant Toxin
|
|---|
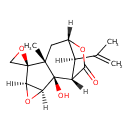
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C15H18O5 |
|---|
| Average Molecular Mass | 278.300 g/mol |
|---|
| Monoisotopic Mass | 278.115 g/mol |
|---|
| CAS Registry Number | 2571-86-0 |
|---|
| IUPAC Name | (1'S,2R,2'R,3'S,5'R,7'R,9'S,12'R)-2'-hydroxy-7'-methyl-12'-(prop-1-en-2-yl)-4',10'-dioxaspiro[oxirane-2,6'-tetracyclo[7.2.1.0²,⁷.0³,⁵]dodecane]-11'-one |
|---|
| Traditional Name | coriamyrtin |
|---|
| SMILES | [H][C@@]12O[C@]1([H])[C@@]1(O)[C@@]3([H])C(=O)O[C@@]([H])(C[C@@]1(C)[C@@]21CO1)[C@@]3([H])C(C)=C |
|---|
| InChI Identifier | InChI=1S/C15H18O5/c1-6(2)8-7-4-13(3)14(5-18-14)10-11(20-10)15(13,17)9(8)12(16)19-7/h7-11,17H,1,4-5H2,2-3H3/t7-,8+,9+,10+,11-,13-,14+,15-/m0/s1 |
|---|
| InChI Key | InChIKey=BWWDLKVKPVKBGJ-TWMZOSGRSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as gamma butyrolactones. Gamma butyrolactones are compounds containing a gamma butyrolactone moiety, which consists of an aliphatic five-member ring with four carbon atoms, one oxygen atom, and bears a ketone group on the carbon adjacent to the oxygen atom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Lactones |
|---|
| Sub Class | Gamma butyrolactones |
|---|
| Direct Parent | Gamma butyrolactones |
|---|
| Alternative Parents | |
|---|
| Substituents | - Oxepane
- Gamma butyrolactone
- Oxane
- Cyclic alcohol
- Tertiary alcohol
- Tetrahydrofuran
- Carboxylic acid ester
- Monocarboxylic acid or derivatives
- Ether
- Oxirane
- Dialkyl ether
- Carboxylic acid derivative
- Oxacycle
- Hydrocarbon derivative
- Organic oxide
- Organooxygen compound
- Organic oxygen compound
- Alcohol
- Carbonyl group
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available | | LogP | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0090000000-0aea41fd56036055c775 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01ti-1290000000-44deb1c9c6ed719dfc4b | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00mo-4930000000-b544a3f2366735da53e8 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0090000000-808669ed82604d0dac0d | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-057i-0090000000-cf71dcb24d4beab7688b | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000x-6960000000-ecf6c832cc8fd5a41c20 | 2016-08-03 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Coriamyrtin has inhibitory activity when modulating receptors of the central nervous system. In other words, coriamyrtin, as a picrotoxin-like sesquiterpene lactones found in Anamirta paniculata and A. cocculus, has a mode of anticonvulsive action as potent transmission inhibitors in the central nervous system. The presence of a hydroxyl group at C-6 could have a significant affinity for a γ-aminobutyric acid (GABA) effector chloride channel, an antagonist to the effects observed on γ-aminobutyric acid but not on alanine or taurine. These experiments were tested on primary afferent terminals and for the antagonism of presynaptic inhibition. Picrotoxin is a known antagonist of GABA-R in all its protein isoforms and R-Gly in the homomeric form. (1) |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| PubChem Compound ID | 442189 |
|---|
| ChEMBL ID | Not Available |
|---|
| ChemSpider ID | 10252019 |
|---|
| KEGG ID | C09379 |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Coriaria myrtifolia |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | - Perez C, Becerra J, Manriquez-Navarro P, Aguayo LG, Fuentealba J, Guzman JL, Joseph-Nathan P, Jimenez V, Munoz MA, Silva M: Inhibitory activities on mammalian central nervous system receptors and computational studies of three sesquiterpene lactones from Coriaria ruscifolia subsp. ruscifolia. Chem Pharm Bull (Tokyo). 2011;59(2):161-5. [21297293 ]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|