| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2014-09-11 02:05:03 UTC |
|---|
| Update Date | 2014-12-24 20:26:55 UTC |
|---|
| Accession Number | T3D4689 |
|---|
| Identification |
|---|
| Common Name | Dacarbazine |
|---|
| Class | Small Molecule |
|---|
| Description | Dacarbazine is only found in individuals that have used or taken this drug. It is an antineoplastic agent. It has significant activity against melanomas. The mechanism of action is not known, but appears to exert cytotoxic effects via its action as an alkylating agent. Other theories include DNA synthesis inhibition by its action as a purine analog, and interaction with SH groups. Dacarbazine is not cell cycle-phase specific. |
|---|
| Compound Type | - Amide
- Amine
- Antineoplastic Agent, Alkylating
- Drug
- Metabolite
- Organic Compound
- Synthetic Compound
|
|---|
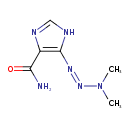
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | 4-(3,3-Dimethyl-1-triazeno)imidazole-5-carboxamide | | 4-(5)-(3,3-Dimethyl-1-triazeno)imidazole-5(4)-carboxamide | | 4-(Dimethyltriazeno)imidazole-5-carboxamide | | 5-(3,3-Dimethyl-1-triazeno)imidazole-4-carboxamide | | 5-(3,3-Dimethyltriazeno)imidazole-4-carboxamide | | 5-(Dimethyltriazeno)imidazole-4-carboxamide | | Biocarbazine | | Biocarbazine R | | Dacarbazin | | Dacarbazina | | Dacarbazinum | | Dacatic | | Déticène | | Detimedac | | DIC | | DTCI | | DTIC | | Dtic-Dome | | Dtic-dome | | DTIE | | HSDB 3219 | | ICDMT | | ICDT | | Imidazole Carboxamide | | NCI-C04717 | | NSC 45388 | | NSC-45388 |
|
|---|
| Chemical Formula | C6H10N6O |
|---|
| Average Molecular Mass | 182.183 g/mol |
|---|
| Monoisotopic Mass | 182.092 g/mol |
|---|
| CAS Registry Number | 4342-03-4 |
|---|
| IUPAC Name | 5-(dimethyltriaz-1-en-1-yl)-1H-imidazole-4-carboxamide |
|---|
| Traditional Name | dacarbazine - dtic |
|---|
| SMILES | CN(C)N=NC1=C(N=CN1)C(N)=O |
|---|
| InChI Identifier | InChI=1S/C6H10N6O/c1-12(2)11-10-6-4(5(7)13)8-3-9-6/h3H,1-2H3,(H2,7,13)(H,8,9) |
|---|
| InChI Key | InChIKey=FDKXTQMXEQVLRF-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 2-heteroaryl carboxamides. 2-heteroaryl carboxamides are compounds containing a heteroaromatic ring that carries a carboxamide group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Carboxylic acid derivatives |
|---|
| Direct Parent | 2-heteroaryl carboxamides |
|---|
| Alternative Parents | |
|---|
| Substituents | - 2-heteroaryl carboxamide
- Imidazole-4-carbonyl group
- Azole
- Imidazole
- Heteroaromatic compound
- Vinylogous amide
- Primary carboxylic acid amide
- Propargyl-type 1,3-dipolar organic compound
- Azacycle
- Organoheterocyclic compound
- Organic 1,3-dipolar compound
- Organic nitrogen compound
- Hydrocarbon derivative
- Organic oxide
- Organooxygen compound
- Organonitrogen compound
- Organopnictogen compound
- Organic oxygen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | - Cytoplasm
- Extracellular
- Membrane
|
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | 205°C | | Boiling Point | Not Available | | Solubility | 4220 mg/L | | LogP | -0.24 |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-002f-7900000000-1b20185f2f8cede43985 | 2017-09-01 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0900000000-19e145e04a29e85dbb78 | 2016-06-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0159-0900000000-46878b8c1d5ef1a88f40 | 2016-06-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-6900000000-7776976c8e2de261cdae | 2016-06-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-1900000000-454c00afd7e71a2e94de | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001c-2900000000-1247d4cc570997f56811 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9400000000-3bc8cd08fb9775dce145 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00lr-0900000000-06f8b85fb44ca1de41ad | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001v-5900000000-bb28672b3383c5c86fda | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-052b-9100000000-43b8467f20b35711585f | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000x-7900000000-45eda8c3304bd65c690f | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-9000000000-d22f5484d8086a357256 | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9000000000-5a148f839c246fca824b | 2021-10-11 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Erratic, slow and incomplete. |
|---|
| Mechanism of Toxicity | The mechanism of action is not known, but appears to exert cytotoxic effects via its action as an alkylating agent. Other theories include DNA synthesis inhibition by its action as a purine analog, and interaction with SH groups. Dacarbazine is not cell cycle-phase specific. |
|---|
| Metabolism | Hepatic.
Route of Elimination: Dacarbazine is subject to renal tubular secretion rather than glomerular filtration. In man, dacarbazine is extensively degraded. Besides unchanged dacarbazine, 5-aminoimidazole -4 carboxamide (AIC) is a major metabolite of dacarbazine excreted in the urine.
Half Life: 5 hours |
|---|
| Toxicity Values | LD50=350mg/kg (orally in mice) |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | 2B, possibly carcinogenic to humans. (1) |
|---|
| Uses/Sources | Dacarbazine is used in the treatment of various cancers, among them malignant melanoma, Hodgkin lymphoma, sarcoma, and islet cell carcinoma of the pancreas. As of mid-2006, dacarbazine is commonly used as a single agent in the treatment of metastatic melanoma, and as part of the ABVD chemotherapy regimen to treat Hodgkin lymphoma, and in the MAID regimen for sarcoma. (Wikipedia) |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB00851 |
|---|
| HMDB ID | HMDB14989 |
|---|
| PubChem Compound ID | Not Available |
|---|
| ChEMBL ID | CHEMBL476 |
|---|
| ChemSpider ID | 10437816 |
|---|
| KEGG ID | Not Available |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | 4305 |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Dacarbazine |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | T3D4689.pdf |
|---|
| General References | - International Agency for Research on Cancer (2014). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. [Link]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|