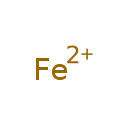| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2014-09-11 02:05:26 UTC |
|---|
| Update Date | 2014-12-24 20:26:55 UTC |
|---|
| Accession Number | T3D4697 |
|---|
| Identification |
|---|
| Common Name | Iron |
|---|
| Class | Small Molecule |
|---|
| Description | A metallic element found in certain minerals, in nearly all soils, and in mineral waters. It is an essential constituent of hemoglobin, cytochrome, and other components of respiratory enzyme systems. Its chief functions are in the transport of oxygen to tissue (hemoglobin) and in cellular oxidation mechanisms. Depletion of iron stores may result in iron-deficiency anemia. Iron is used to build up the blood in anemia. |
|---|
| Compound Type | - Anti-Anemic Agent
- Drug
- Food Toxin
- Household Toxin
- Inorganic Compound
- Metabolite
- Metal
- Supplement
- Synthetic Compound
- Trace Element
|
|---|
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | 26Fe | | Ed-In-Sol | | Eisen | | Fe | | Fe-40 | | Feosol | | Feostat | | fer | | Fer-In-Sol | | Feraheme | | Feratab | | Ferate | | Fergon | | Ferralet | | Ferretts | | Ferro sanol | | Ferro-Caps | | Ferro-Time | | Ferrousal | | Ferrum | | Hierro | | Siderol | | Simron | | Slow Fe | | Vitedyn-Slo | | Yieronia |
|
|---|
| Chemical Formula | Fe |
|---|
| Average Molecular Mass | 55.845 g/mol |
|---|
| Monoisotopic Mass | 55.935 g/mol |
|---|
| CAS Registry Number | 7439-89-6 |
|---|
| IUPAC Name | lambda2-iron(2+) ion |
|---|
| Traditional Name | lambda2-iron(2+) ion |
|---|
| SMILES | [Fe] |
|---|
| InChI Identifier | InChI=1S/Fe |
|---|
| InChI Key | InChIKey=XEEYBQQBJWHFJM-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of inorganic compounds known as homogeneous transition metal compounds. These are inorganic compounds containing only metal atoms,with the largest atom being a transition metal atom. |
|---|
| Kingdom | Inorganic compounds |
|---|
| Super Class | Homogeneous metal compounds |
|---|
| Class | Homogeneous transition metal compounds |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Homogeneous transition metal compounds |
|---|
| Alternative Parents | Not Available |
|---|
| Substituents | - Homogeneous transition metal
|
|---|
| Molecular Framework | Not Available |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Endogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | 1538°C | | Boiling Point | Not Available | | Solubility | Not Available | | LogP | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-9000000000-af3e7aec4f5bd9668683 | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-9000000000-af3e7aec4f5bd9668683 | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-9000000000-af3e7aec4f5bd9668683 | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-9000000000-3335fec4c3184739b75e | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-9000000000-3335fec4c3184739b75e | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0udi-9000000000-3335fec4c3184739b75e | 2016-08-03 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | The efficiency of absorption depends on the salt form, the amount administered, the dosing regimen and the size of iron stores. Subjects with normal iron stores absorb 10% to 35% of an iron dose. Those who are iron deficient may absorb up to 95% of an iron dose. |
|---|
| Mechanism of Toxicity | Iron is necessary for the production of hemoglobin. Iron-deficiency can lead to decreased production of hemoglobin and a microcytic, hypochromic anemia. |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | In a young child, 75 milligrams per kilogram is considered extremely dangerous. A dose of 30 milligrams per kilogram can lead to symptoms of toxicity. A peak serum iron concentration of five micrograms or more per ml is associated with moderate to severe poisoning in many. |
|---|
| Lethal Dose | Estimates of a lethal dosage range from 180 milligrams per kilogram and upwards. |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | Used in preventing and treating iron-deficiency anemia. |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB01592 |
|---|
| HMDB ID | HMDB15531 |
|---|
| PubChem Compound ID | 23925 |
|---|
| ChEMBL ID | Not Available |
|---|
| ChemSpider ID | 22368 |
|---|
| KEGG ID | C00023 |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | 18248 |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Iron |
|---|
| References |
|---|
| Synthesis Reference | Walter Lugscheider, Paul Mullner, Wilhelm Schiffer, Alois Leutgob, “Arrangement for producing metals, such as molten pig iron, steel pre-material and ferroalloys.” U.S. Patent US4617671, issued 0000. |
|---|
| MSDS | Link |
|---|
| General References | Not Available |
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|