| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2014-09-11 02:05:38 UTC |
|---|
| Update Date | 2014-12-24 20:26:55 UTC |
|---|
| Accession Number | T3D4702 |
|---|
| Identification |
|---|
| Common Name | Melphalan |
|---|
| Class | Small Molecule |
|---|
| Description | An alkylating nitrogen mustard that is used as an antineoplastic in the form of the levo isomer - melphalan, the racemic mixture - merphalan, and the dextro isomer - medphalan; toxic to bone marrow, but little vesicant action; potential carcinogen. |
|---|
| Compound Type | - Amine
- Drug
- Metabolite
- Organic Compound
- Organochloride
- Synthetic Compound
|
|---|
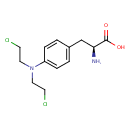
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | 3-(P-(Bis(2-chloroethyl)amino)phenyl)-L-alanine | | 3-P-(Di(2-chloroethyl)amino)-phenyl-L-alanine | | 4-(Bis(2-chloroethyl)amino)-L-phenylalanine | | Alkeran | | L-3-(P-(Bis(2-chloroethyl)amino)phenyl)alanine | | L-PAM | | L-Phenylalanine mustard | | L-Sarcolysine | | Melfalano | | Melphalanum | | p-Bis(beta-chloroethyl)aminophenylalanine | | P-Di-(2-chloroethyl)amino-L-phenylalanine | | P-L-Sarcolysin | | p-N,N-bis(2-chloroethyl)amino-L-phenylalanine | | P-N-Bis(2-chloroethyl)amino-L-phenylalanine | | Phenylalanine mustard | | Phenylalanine nitrogen mustard |
|
|---|
| Chemical Formula | C13H18Cl2N2O2 |
|---|
| Average Molecular Mass | 305.200 g/mol |
|---|
| Monoisotopic Mass | 304.075 g/mol |
|---|
| CAS Registry Number | 148-82-3 |
|---|
| IUPAC Name | (2S)-2-amino-3-{4-[bis(2-chloroethyl)amino]phenyl}propanoic acid |
|---|
| Traditional Name | melphalan |
|---|
| SMILES | [H][C@](N)(CC1=CC=C(C=C1)N(CCCl)CCCl)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C13H18Cl2N2O2/c14-5-7-17(8-6-15)11-3-1-10(2-4-11)9-12(16)13(18)19/h1-4,12H,5-9,16H2,(H,18,19)/t12-/m0/s1 |
|---|
| InChI Key | InChIKey=SGDBTWWWUNNDEQ-LBPRGKRZSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as phenylalanine and derivatives. Phenylalanine and derivatives are compounds containing phenylalanine or a derivative thereof resulting from reaction of phenylalanine at the amino group or the carboxy group, or from the replacement of any hydrogen of glycine by a heteroatom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Phenylalanine and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Phenylalanine or derivatives
- 3-phenylpropanoic-acid
- Alpha-amino acid
- Amphetamine or derivatives
- L-alpha-amino acid
- Nitrogen mustard
- Tertiary aliphatic/aromatic amine
- Aniline or substituted anilines
- Dialkylarylamine
- Aralkylamine
- Monocyclic benzene moiety
- Benzenoid
- Amino acid
- Tertiary amine
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Organic oxide
- Organooxygen compound
- Organonitrogen compound
- Organochloride
- Organohalogen compound
- Hydrocarbon derivative
- Organic nitrogen compound
- Primary aliphatic amine
- Primary amine
- Carbonyl group
- Organopnictogen compound
- Alkyl halide
- Amine
- Organic oxygen compound
- Alkyl chloride
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | - Cytoplasm
- Extracellular
- Membrane
|
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | 182.5°C | | Boiling Point | Not Available | | Solubility | < 0.1 g/100 mL at 22°C | | LogP | -0.52 |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-052f-3890000000-153ff87813e7a797c73b | 2017-09-01 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-01c3-8295000000-421b0e52574345db725f | 2017-10-06 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-qTof , Positive | splash10-000w-2930000000-b95beb5e9c3e61b7687f | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-000w-2930000000-b95beb5e9c3e61b7687f | 2017-09-14 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0093000000-78045bae5e6c1e79e22d | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-1490000000-3b1f7009692b1139989b | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-02t9-3930000000-b0f45ffda87388d4384a | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0049000000-8c401366ace5d7fc1f08 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0v4i-1192000000-fd136d877921c8afed95 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00di-9530000000-511158435cb1ef063085 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0095000000-3f3242a1d9debe0c8315 | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00bi-0090000000-91067233804f48cd760a | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014j-0930000000-af3e37be6b85ec7f144b | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-7090000000-6793f4f7ed0a2c22f972 | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-9000000000-c2fa753da65a4bac80a1 | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001i-9000000000-ed9c052b92676bc8a30b | 2021-10-11 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Incomplete, variable, 25-89% post oral dose |
|---|
| Mechanism of Toxicity | Alkylating agents work by three different mechanisms: 1) attachment of alkyl groups to DNA bases (primarily at the N-7 position of guanine and to a lesser extent, at the N-3 position of adenine), forming monoadducts and resulting in the DNA being fragmented by repair enzymes in their attempts to replace the alkylated bases, preventing DNA synthesis and RNA transcription from the affected DNA, 2) DNA damage via the formation of cross-links (bonds between atoms in the DNA) which prevents DNA from being separated for synthesis or transcription, and 3) the induction of mispairing of the nucleotides leading to mutations. |
|---|
| Metabolism | Melphalan is not actively metabolised, it spontaneously degrades to mono and dihydroxy products.
Route of Elimination: The 24-hour urinary excretion of parent drug in these patients was 10% Њ± 4.5%, suggesting that renal clearance is not a major route of elimination of parent drug.
Half Life: 1.5 (±0.83) hours |
|---|
| Toxicity Values | LD50=11.2 mg/kg (orally in rat) |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | 1, carcinogenic to humans. (4) |
|---|
| Uses/Sources | For the palliative treatment of multiple myeloma and for the palliation of non-resectable epithelial carcinoma of the ovary. Has also been used alone or as part of various chemotherapeutic regimens as an adjunct to surgery in the treatment of breast cancer, alone or in combination regimens for palliative treatment of locally recurrent or unresectable in-transit metastatic melanoma of the extremities, as well as for the treatment of amyloidosis with prednisone. |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | The principal toxic effect is bone marrow suppression. |
|---|
| Symptoms | Vomiting, ulceration of the mouth, diarrhea, and hemorrhage of the gastrointestinal tract. |
|---|
| Treatment | Not Available |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB01042 |
|---|
| HMDB ID | HMDB15176 |
|---|
| PubChem Compound ID | 460612 |
|---|
| ChEMBL ID | CHEMBL852 |
|---|
| ChemSpider ID | 405297 |
|---|
| KEGG ID | C07122 |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | 28876 |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Melphalan |
|---|
| References |
|---|
| Synthesis Reference | DrugSyn.org |
|---|
| MSDS | T3D4702.pdf |
|---|
| General References | - Loeber R, Michaelson E, Fang Q, Campbell C, Pegg AE, Tretyakova N: Cross-linking of the DNA repair protein Omicron6-alkylguanine DNA alkyltransferase to DNA in the presence of antitumor nitrogen mustards. Chem Res Toxicol. 2008 Apr;21(4):787-95. doi: 10.1021/tx7004508. Epub 2008 Feb 14. [18324787 ]
- Souliotis VL, Dimopoulos MA, Episkopou HG, Kyrtopoulos SA, Sfikakis PP: Preferential in vivo DNA repair of melphalan-induced damage in human genes is greatly affected by the local chromatin structure. DNA Repair (Amst). 2006 Aug 13;5(8):972-85. Epub 2006 Jun 15. [16781199 ]
- Moscow JA, Swanson CA, Cowan KH: Decreased melphalan accumulation in a human breast cancer cell line selected for resistance to melphalan. Br J Cancer. 1993 Oct;68(4):732-7. [8398701 ]
- International Agency for Research on Cancer (2014). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. [Link]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|