| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2014-09-11 05:18:36 UTC |
|---|
| Update Date | 2014-12-24 20:26:58 UTC |
|---|
| Accession Number | T3D4837 |
|---|
| Identification |
|---|
| Common Name | Geranyl acetate |
|---|
| Class | Small Molecule |
|---|
| Description | Neryl acetate is found in cardamom. Neryl acetate is found in citrus, kumquat and pummelo peel oils, ginger, cardamon, clary sage, myrtle leaf and myrtle berries. Neryl acetate is a flavouring agent.
Geranyl acetate belongs to the family of Fatty Alcohol Esters. These are ester derivatives of a fatty alcohol. |
|---|
| Compound Type | - Ester
- Ether
- Flavouring Agent
- Food Toxin
- Household Toxin
- Metabolite
- Natural Compound
- Organic Compound
- Plant Toxin
|
|---|
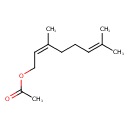
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | (2E)-3,7-Dimethyl-2,6-octadienyl acetate | | (2E)-3,7-dimethylocta-2,6-dien-1-yl acetate | | (E)-3,7-Dimethyl-2,6-octadien-1-yl acetate | | (Z)-3,7-Dimethyl-2,6-octadienyl acetate | | 1,6-Octadiene, 7-methyl-3-methylene-, acetylated | | 1-Octanol, 3,7-dimethyl-, 1-acetate, tetradehydro deriv. | | 1-Octanol, 3,7-dimethyl-, acetate, tetradehydro deriv. | | 2,6-Dimethyl-2,6-octadiene-8-yl acetate | | 2,6-Octadien-1-ol, 3,7-dimethyl-, acetate | | 3,7-Dimethyl-1-acetate(2E)-2,6-Octadien-1-ol | | 3,7-Dimethyl-1-acetate(2Z)-2,6-Octadien-1-ol | | 3,7-Dimethyl-2,6-octadien-1-ol acetate | | 3,7-Dimethyl-acetate(2E)-2,6-Octadien-1-ol | | 3,7-Dimethyl-acetate(2Z)-2,6-Octadien-1-ol | | 3,7-Dimethyl-acetate(E)-2,6-Octadien-1-ol | | 3,7-Dimethyl-acetatetrans-2,6-Octadien-1-ol | | 3,7-Dimethyloctyl acetate, tetradehydro derivative | | Acetic acid, geraniol ester | | Acetic acid, geranyl ester | | cis-3,7-Dimethyl-2,6-octadien-1-ol acetate | | cis-3,7-Dimethyl-2,6-octadien-1-yl acetate | | cis-3,7-Dimethyl-2,6-octadien-1-yl ethanoate | | cis-Geranyl acetate | | FEMA 2509 | | Geranyl acetate a | | Geranyl acetic acid | | Geranyl ethanoate | | Meraneine | | Nerol acetate (6CI) | | Neryl ethanoate | | trans-2,6-Dimethyl-2,6-octadien-8-yl ethanoate | | trans-3,7-Dimethyl-2,6-octadien-1-yl acetate | | trans-3,7-Dimethyl-2,6-octadien-1-yl ethanoate | | trans-3,7-Dimethyl-2,6-octadienyl acetate | | Trans-geraniol acetate | | Trans-geranyl acetate |
|
|---|
| Chemical Formula | C12H20O2 |
|---|
| Average Molecular Mass | 196.286 g/mol |
|---|
| Monoisotopic Mass | 196.146 g/mol |
|---|
| CAS Registry Number | 105-87-3 |
|---|
| IUPAC Name | (2Z)-3,7-dimethylocta-2,6-dien-1-yl acetate |
|---|
| Traditional Name | neryl acetate |
|---|
| SMILES | [H]\C(COC(C)=O)=C(/C)CCC=C(C)C |
|---|
| InChI Identifier | InChI=1S/C12H20O2/c1-10(2)6-5-7-11(3)8-9-14-12(4)13/h6,8H,5,7,9H2,1-4H3/b11-8- |
|---|
| InChI Key | InChIKey=HIGQPQRQIQDZMP-FLIBITNWSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as fatty alcohol esters. These are ester derivatives of a fatty alcohol. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Fatty alcohol esters |
|---|
| Direct Parent | Fatty alcohol esters |
|---|
| Alternative Parents | |
|---|
| Substituents | - Fatty alcohol ester
- Monoterpenoid
- Acyclic monoterpenoid
- Carboxylic acid ester
- Monocarboxylic acid or derivatives
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Liquid |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | < 25 °C | | Boiling Point | Not Available | | Solubility | Not Available | | LogP | 4.04 |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-014l-9100000000-93c6abc7985ec7f0a488 | 2017-09-12 | View Spectrum | | GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-00ko-9500000000-cdf126f2c6e288b8761f | 2017-09-12 | View Spectrum | | GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-014l-9100000000-867992beb9e7c804a69d | 2017-09-12 | View Spectrum | | GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-014l-9100000000-93c6abc7985ec7f0a488 | 2018-05-18 | View Spectrum | | GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-00ko-9500000000-cdf126f2c6e288b8761f | 2018-05-18 | View Spectrum | | GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-014l-9100000000-867992beb9e7c804a69d | 2018-05-18 | View Spectrum | | GC-MS | GC-MS Spectrum - GC-EI-Q (Non-derivatized) | splash10-014l-9100000000-b15d9b4b6de3799488d0 | 2020-07-08 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00kf-9400000000-f7513133120d5e44712a | 2017-09-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000b-1900000000-e07e2965d977a6e2825d | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-5900000000-e5f05dabacd073dce84c | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0gbc-9100000000-4932e3e742f871bd9300 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-4900000000-7a2e3b89cdc43f9c6174 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-9300000000-3c0cda66ebfc365b1b71 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4l-9300000000-02983c019224fd67a6c7 | 2016-08-03 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB35157 |
|---|
| PubChem Compound ID | 1549025 |
|---|
| ChEMBL ID | CHEMBL2268549 |
|---|
| ChemSpider ID | 1266018 |
|---|
| KEGG ID | C09861 |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Geranyl_acetate |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | T3D4837.pdf |
|---|
| General References | - Yannai, Shmuel. (2004) Dictionary of food compounds with CD-ROM: Additives, flavors, and ingredients. Boca Raton: Chapman & Hall/CRC.
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|