| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2009-06-19 21:58:21 UTC |
|---|
| Update Date | 2014-12-24 20:23:12 UTC |
|---|
| Accession Number | T3D1138 |
|---|
| Identification |
|---|
| Common Name | Methylcyclopentadienyl manganese tricarbonyl |
|---|
| Class | Small Molecule |
|---|
| Description | Methylcyclopentadienyl manganese tricarbonyl (MMT) is a chemical compound of manganese. It was initially used as a supplement to the gasoline additive tetraethyl lead to increase a fuel's octane rating, then later also used in unleaded gasoline. It has been banned in various countries in the past due to its toxicity, but is available in most places today, despite controvery over its safety. Manganese is a naturally occurring metal with the symbol Mn and the atomic number 25. It does not occur naturally in its pure form, but is found in many types of rocks in combination with other substances such as oxygen, sulfur, or chlorine. Manganese occurs naturally in most foods and small amounts are needed to stay healthy, as manganese ions act as cofactors for a number of enzymes. (4, 5, 6) |
|---|
| Compound Type | - Amine
- Food Toxin
- Household Toxin
- Manganese Compound
- Organic Compound
- Organometallic
- Pollutant
- Synthetic Compound
|
|---|
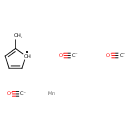
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | (1-Methyl-2,4-cyclopentadien-1-yl)manganese tricarbonyl | | (methylcyclopentadienyl)manganese tricarbonyl | | (methylcyclopentadienyl)tricarbonylmanganese | | 2-(Methylcyclopentadienyl)manganesetricarbonyl | | 2-Methylcyclopentadienyl manganese tricarbonyl | | 2-Methylcyclopentadienylmanganese tricarbonyl | | Antiknock-33 | | Combustion improver -2 | | Manganese methyl cyclopentadienyl tricarbonyl | | Manganese, tricarbonyl methylcyclopentadienyl | | Manganese, tricarbonyl(methyl-pi-cyclopentadienyl)- (8CI) | | Manganese, tricarbonylmethylcyclopentadienyl | | Manganese, tricarbonyl[(1,2,3,4,5-.eta.)-1-methyl-2,4 | | Methyl cyclopentadienyl manganese tricarbonyl | | Methylcyklopentadientrikarbonylmanganium | | Methylcymantrene | | MMT | | Pi-(methylcyclopentadienyl)manganese tricarbonyl | | Pi-methylcyclopentadienylmanganese tricarbonyl | | Tricarbonyl(2-methylcyclopentadienyl)manganese | | Tricarbonyl(eta(5)-methylcyclopentadienyl)manganese | | Tricarbonyl(methylcyclopentadienyl)manganese |
|
|---|
| Chemical Formula | C9H7MnO3 |
|---|
| Average Molecular Mass | 218.088 g/mol |
|---|
| Monoisotopic Mass | 217.978 g/mol |
|---|
| CAS Registry Number | 12108-13-3 |
|---|
| IUPAC Name | 2-methylcyclopenta-2,4-dien-1-yl tris(methanidylidyneoxidanium) manganese |
|---|
| Traditional Name | 2-methylcyclopenta-2,4-dien-1-yl tris(carbon monoxide) manganese |
|---|
| SMILES | [Mn].[C-]#[O+].[C-]#[O+].[C-]#[O+].CC1=CC=C[CH]1 |
|---|
| InChI Identifier | InChI=1S/C6H7.3CO.Mn/c1-6-4-2-3-5-6;3*1-2;/h2-5H,1H3;;;; |
|---|
| InChI Key | InChIKey=LYHJNAIHGFWRKM-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as cycloalkenes. These are unsaturated monocyclic hydrocarbons having one endocyclic double bond. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Hydrocarbons |
|---|
| Class | Unsaturated hydrocarbons |
|---|
| Sub Class | Olefins |
|---|
| Direct Parent | Cycloalkenes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Organic transition metal salt
- Cycloalkene
- Organic oxygen compound
- Unsaturated aliphatic hydrocarbon
- Hydrocarbon derivative
- Organic salt
- Aliphatic homomonocyclic compound
|
|---|
| Molecular Framework | Not Available |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Liquid |
|---|
| Appearance | Yellow to dark orange liquid. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | 1.5°C | | Boiling Point | Not Available | | Solubility | 0.029 mg/mL at 25°C [GARRISON,AW et al. (1995)] | | LogP | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0090000000-618af413accb30414c94 | 2016-06-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-0090000000-618af413accb30414c94 | 2016-06-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014i-0090000000-618af413accb30414c94 | 2016-06-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0090000000-f9b84666ff8e388846d9 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-0090000000-f9b84666ff8e388846d9 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014i-0090000000-f9b84666ff8e388846d9 | 2016-08-03 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Oral (4) ; inhalation (4) |
|---|
| Mechanism of Toxicity | Manganese is a cellular toxicant that can impair transport systems, enzyme activities, and receptor functions. It primarily targets the central nervous system, particularily the globus pallidus of the basal ganglia. It is believed that the manganese ion, Mn(II), enhances the autoxidation or turnover of various intracellular catecholamines, leading to increased production of free radicals, reactive oxygen species, and other cytotoxic metabolites, along with a depletion of cellular antioxidant defense mechanisms, leading to oxidative damage and selective destruction of dopaminergic neurons. In addition to dopamine, manganese is thought to perturbations other neurotransmitters, such as GABA and glutamate. In order to produce oxidative damage, manganese must first overwhelm the antioxidant enzyme manganese superoxide dismutase. The neurotoxicity of Mn(II) has also been linked to its ability to substitute for Ca(II) under physiological conditions. It can enter mitochondria via the calcium uniporter and inhibit mitochondrial oxidative phosphorylation. It may also inhibit the efflux of Ca(II), which can result in a loss of mitochondrial membrane integrity. Mn(II) has been shown to inhibit mitochondrial aconitase activity to a significant level, altering amino acid metabolism and cellular iron homeostasis. (4) |
|---|
| Metabolism | Manganese is absorbed mainly via ingestion, but can also be inhaled. It binds to alpha-2-macroglobulin, albumin, or transferrin in the plasma and is distributed to the brain and all other mammalian tissues, though it tends to accumulate more in the liver, pancreas, and kidney. MMT is metabolized by cytochrome P-450 enzymes into hydroxylmethylcyclopentadienyl
manganese tricarbonyl and carboxycyclopentadienyl manganese tricarbonyl. These metabolites are excreted in the urine and faeces. (4) |
|---|
| Toxicity Values | LD50: 140-795 mg/kg (Dermal, Rabbit) (2)
LD50: 58 mg/kg (Oral, Rat) (1)
LC50: 247 mg/m3 over 1 hour (Inhalation, Rat) (2) |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | Methylcyclopentadienyl manganese tricarbonyl (MMT) is a chemical compound of manganese. It was initially used as a supplement to the gasoline additive tetraethyl lead to increase a fuel's octane rating, then later also used in unleaded gasoline. It has been banned in various countries in the past due to its toxicity, but is available in most places today, despite controvery over its safety. (6) |
|---|
| Minimum Risk Level | Chronic Inhalation: 0.0003 mg/m3 (3) |
|---|
| Health Effects | Manganese mainly affects the nervous system and may cause behavioral changes and other nervous system effects, which include movements that may become slow and clumsy. This combination of symptoms when sufficiently severe is referred to as “manganism”. (4) |
|---|
| Symptoms | Manganese mainly affects the nervous system and may cause behavioral changes and other nervous system effects, which include movements that may become slow and clumsy. This combination of symptoms when sufficiently severe is referred to as “manganism”. (4) |
|---|
| Treatment | Not Available |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| PubChem Compound ID | 25511 |
|---|
| ChEMBL ID | Not Available |
|---|
| ChemSpider ID | Not Available |
|---|
| KEGG ID | Not Available |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | Not Available |
|---|
| BioCyc ID | MN%2b3 |
|---|
| CTD ID | C009907 |
|---|
| Stitch ID | Methylcyclopentadienyl manganese tricarbonyl |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | 4267 |
|---|
| Wikipedia Link | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | T3D1138.pdf |
|---|
| General References | - Gong P, Kuperman RG, Sunahara GI: Genotoxicity of 2,4- and 2,6-dinitrotoluene as measured by the Tradescantia micronucleus (Trad-MCN) bioassay. Mutat Res. 2003 Jul 8;538(1-2):13-8. [12834750 ]
- Verschueren K (1983). Handbook of Environmental Data of Organic Chemicals. 2nd ed. New York, NY: Van Nostrand Reinhold Co.
- ATSDR - Agency for Toxic Substances and Disease Registry (2001). Minimal Risk Levels (MRLs) for Hazardous Substances. U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). [Link]
- ATSDR - Agency for Toxic Substances and Disease Registry (2008). Toxicological profile for manganese. U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). [Link]
- Wikipedia. Manganese. Last Updated 26 May 2009. [Link]
- Wikipedia. Methylcyclopentadienyl manganese tricarbonyl. Last Updated 9 May 2009. [Link]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|