| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2009-06-19 21:58:51 UTC |
|---|
| Update Date | 2014-12-24 20:23:59 UTC |
|---|
| Accession Number | T3D1486 |
|---|
| Identification |
|---|
| Common Name | Aluminium chlorohydrate |
|---|
| Class | Small Molecule |
|---|
| Description | Aluminium chlorohydrate is a group of specific aluminium salts having the general formula AlnCl(3n-m)(OH)m. It is used in deodorants and antiperspirants and as a coagulant in water purification. The Food and Drug Administration considers the use of aluminium chlorohydrate in antiperspirants to be safe and it is permitted in concentrations up to 25%. Aluminium chlorohydrate is concerned with Alzheimer's disease and breast cancer. |
|---|
| Compound Type | - Aluminum Compound
- Food Toxin
- Household Toxin
- Inorganic Compound
- Synthetic Compound
|
|---|
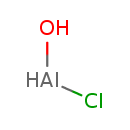
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | Aloxicoll | | Aluminium chlorohydric acid | | Aluminol ach | | Aluminum chlorhydrate | | Aluminum chlorhydroxide | | Aluminum chloride hydroxide | | Aluminum chloride hydroxide oxide, basic | | Aluminum chloride oxide | | Aluminum chloride, basic | | Aluminum chlorohydrate (anhydrous) | | Aluminum chlorohydrol | | Aluminum hydroxide chloride | | Aluminum hydroxychloride | | Aluminum oxychloride | | Aquarhone 18 | | Astringen | | Astringen 10 | | Banoltan white | | Basic aluminum chloride | | Basic aluminum chloride, hydrate | | Berukotan ac-p | | Cartafix la | | Cawood 5025 | | Chlorhydrol | | Chlorhydrol micro-DRY | | Chlorhydrol micro-DRY suf | | Dialuminium-chlorid-pentahydroxid | | E 200 (coagulant) | | Gelsica | | Hessidrex WT | | Hydral | | Hydrofugal | | Kempac 10 | | Kempac 20 | | Kemwater PAX 14 | | Locron | | Locron p | | Locron s | | OCAL | | Oulupac 180 | | PAC | | Pac (salt) | | PAC 250AD | | PACK 300M | | Paho 2S | | PALC | | Sansudor | | Wickenol cps 325 |
|
|---|
| Chemical Formula | AlClH2O |
|---|
| Average Molecular Mass | 80.450 g/mol |
|---|
| Monoisotopic Mass | 79.961 g/mol |
|---|
| CAS Registry Number | 1327-41-9 |
|---|
| IUPAC Name | chloroalumanol |
|---|
| Traditional Name | chloroalumanol |
|---|
| SMILES | O[AlH]Cl |
|---|
| InChI Identifier | InChI=1S/Al.ClH.H2O.H/h;1H;1H2;/q+2;;;/p-2 |
|---|
| InChI Key | InChIKey=MTSHNTORJWMHCG-UHFFFAOYSA-L |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of inorganic compounds known as post-transition metal chlorides. These are inorganic compounds in which the largest halogen atom is Chlorine, and the heaviest metal atom is a post-transition metal. |
|---|
| Kingdom | Inorganic compounds |
|---|
| Super Class | Mixed metal/non-metal compounds |
|---|
| Class | Post-transition metal salts |
|---|
| Sub Class | Post-transition metal chlorides |
|---|
| Direct Parent | Post-transition metal chlorides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Post-transition metal chloride
- Inorganic chloride salt
- Inorganic salt
|
|---|
| Molecular Framework | Not Available |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available | | LogP | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-9000000000-afa3c161bea593a5c465 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-9000000000-afa3c161bea593a5c465 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000i-9000000000-afa3c161bea593a5c465 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-9000000000-b643e10f75dd6817b08b | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-9000000000-b643e10f75dd6817b08b | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001i-9000000000-b643e10f75dd6817b08b | 2016-08-03 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Oral (6) ; inhalation (6) |
|---|
| Mechanism of Toxicity | The main target organs of aluminum are the central nervous system and bone. Aluminum binds with dietary phosphorus and impairs gastrointestinal absorption of phosphorus. The decreased phosphate body burden results in osteomalacia (softening of the bones due to defective bone mineralization) and rickets. Aluminum's neurotoxicity is believed to involve several mechanisms. Changes in cytoskeletal protein functions as a results of altered phosphorylation, proteolysis, transport, and synthesis are believed to be one cause. Aluminum may induce neurobehavioral effects by affecting permeability of the blood-brain barrier, cholinergic activity, signal transduction pathways, lipid peroxidation, and impair neuronal glutamate nitric oxide-cyclic GMP pathway, as well as interfere with metabolism of essential trace elements because of similar coordination chemistries and consequent competitive interactions. It has been suggested that aluminum's interaction with estrogen receptors increases the expression of estrogen-related genes and thereby contributes to the progression of breast cancer (1), but studies have not been able to establish a clear link between aluminum and increased risk of breast cancer (3). Certain aluminum salts induce immune responses by activating inflammasomes. (6, 1, 2) |
|---|
| Metabolism | Aluminum is poorly absorbed following either oral or inhalation exposure and is essentially not absorbed dermally. The bioavailability of aluminum is strongly influenced by the aluminum compound and the presence of dietary constituents which can complex with aluminum and enhance or inhibit its absorption. Aluminum binds to various ligands in the blood and distributes to every organ, with highest concentrations found in bone and lung tissues. In living organisms, aluminum is believed to exist in four different forms: as free ions, as low-molecular-weight complexes, as physically bound macromolecular complexes, and as covalently bound macromolecular complexes. Absorbed aluminum is excreted principally in the urine and, to a lesser extent, in the bile, while unabsorbed aluminum is excreted in the faeces. (6) |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not listed by IARC. IARC classified aluminum production as carcinogenic to humans (Group 1), but did not implicate aluminum itself as a human carcinogen. (9) A link between use of aluminum-containing antiperspirants and increased risk of breast cancer has been proposed (1), but studies have not been able to establish a clear link (3). |
|---|
| Uses/Sources | Aluminium chlorohydrate is used in deodorants and antiperspirants and as a flocculant in water purification. (8) |
|---|
| Minimum Risk Level | Intermediate Oral: 1.0 mg/kg/day (5)
Chronic Oral: 1.0 mg/kg/day (5) |
|---|
| Health Effects | Aluminum targets the nervous system and causes decreased nervous system performance and is associated with altered function of the blood-brain barrier. The accumulation of aluminum in the body may cause bone or brain diseases. High levels of aluminum have been linked to Alzheimer's disease. A small percentage of people are allergic to aluminium and experience contact dermatitis, digestive disorders, vomiting or other symptoms upon contact or ingestion of products containing aluminium. (6, 7) |
|---|
| Symptoms | Inhalating aluminum dust causes coughing and abnormal chest X-rays. A small percentage of people are allergic to aluminium and experience contact dermatitis, digestive disorders, vomiting or other symptoms upon contact or ingestion of products containing aluminium. (6, 7) |
|---|
| Treatment | EYES: irrigate opened eyes for several minutes under running water. INGESTION: do not induce vomiting. Rinse mouth with water (never give anything by mouth to an unconscious person). Seek immediate medical advice. SKIN: should be treated immediately by rinsing the affected parts in cold running water for at least 15 minutes, followed by thorough washing with soap and water. If necessary, the person should shower and change contaminated clothing and shoes, and then must seek medical attention. INHALATION: supply fresh air. If required provide artificial respiration. |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| PubChem Compound ID | Not Available |
|---|
| ChEMBL ID | Not Available |
|---|
| ChemSpider ID | 21781843 |
|---|
| KEGG ID | Not Available |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | C016213 |
|---|
| Stitch ID | Aluminium chlorohydrate |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | 10652 |
|---|
| Wikipedia Link | Aluminium_chlorohydrate |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | T3D1486.pdf |
|---|
| General References | - Darbre PD: Metalloestrogens: an emerging class of inorganic xenoestrogens with potential to add to the oestrogenic burden of the human breast. J Appl Toxicol. 2006 May-Jun;26(3):191-7. [16489580 ]
- Aimanianda V, Haensler J, Lacroix-Desmazes S, Kaveri SV, Bayry J: Novel cellular and molecular mechanisms of induction of immune responses by aluminum adjuvants. Trends Pharmacol Sci. 2009 Jun;30(6):287-95. doi: 10.1016/j.tips.2009.03.005. Epub 2009 May 11. [19439372 ]
- Willhite CC, Karyakina NA, Yokel RA, Yenugadhati N, Wisniewski TM, Arnold IM, Momoli F, Krewski D: Systematic review of potential health risks posed by pharmaceutical, occupational and consumer exposures to metallic and nanoscale aluminum, aluminum oxides, aluminum hydroxide and its soluble salts. Crit Rev Toxicol. 2014 Oct;44 Suppl 4:1-80. doi: 10.3109/10408444.2014.934439. [25233067 ]
- WHO (1997). Environmental Health Criteria 194: Aluminum.
- ATSDR - Agency for Toxic Substances and Disease Registry (2001). Minimal Risk Levels (MRLs) for Hazardous Substances. U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). [Link]
- ATSDR - Agency for Toxic Substances and Disease Registry (2008). Toxicological profile for aluminum. U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). [Link]
- Wikipedia. Aluminium. Last Updated 16 June 2009. [Link]
- Wikipedia. Aluminium chlorohydrate. Last Updated 22 May 2009. [Link]
- International Agency for Research on Cancer (2014). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. [Link]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|