| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2009-06-24 17:25:21 UTC |
|---|
| Update Date | 2014-12-24 20:25:06 UTC |
|---|
| Accession Number | T3D2040 |
|---|
| Identification |
|---|
| Common Name | Pentabromodiphenyl ethers |
|---|
| Class | Small Molecule |
|---|
| Description | 1,2,4-Tribromo-5-(2,4-dibromophenoxy)benzene is classified as a Food Contaminant (code WG) in the DFC. |
|---|
| Compound Type | - Aromatic Hydrocarbon
- Bromide Compound
- Ether
- Food Toxin
- Metabolite
- Organic Compound
- Organobromide
- Polybrominated Biphenyl
- Synthetic Compound
|
|---|
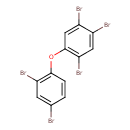
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | 1,2,4-Tribromo-5-(2,4-dibromophenoxy)benzene | | 2,2',4,4',5-PentaBDE | | 2,2',4,4',5-Pentabromodiphenyl ether | | 2,2',4,4',5-Pentabromodiphenyl ether solution | | BDE 99 | | BDE No 99 solution | | Benzene, 1,1'-oxybis-, pentabromo deriv | | Benzene, 1,1'-oxybis-, pentabromo deriv. | | Bromkal G 1 | | Diphenyl ether, pentabromo derivative | | PBDE 99 | | Pentabromodiphenyl ether | | Pentabromodiphenyl oxide | | Pentabromodiphenyl oxide (technical) | | Pentabromodiphenyl oxide (technical) (DE 71) | | Pentabromodiphenyl oxide repackaged | | Pentabromophenoxybenzene | | Planelon PB 501 | | Saytex 125 |
|
|---|
| Chemical Formula | C12H5Br5O |
|---|
| Average Molecular Mass | 564.688 g/mol |
|---|
| Monoisotopic Mass | 559.626 g/mol |
|---|
| CAS Registry Number | 32534-81-9 |
|---|
| IUPAC Name | 1,2,4-tribromo-5-(2,4-dibromophenoxy)benzene |
|---|
| Traditional Name | pentabromodiphenyl ether |
|---|
| SMILES | BrC1=CC(Br)=C(OC2=C(Br)C=C(Br)C(Br)=C2)C=C1 |
|---|
| InChI Identifier | InChI=1S/C12H5Br5O/c13-6-1-2-11(9(16)3-6)18-12-5-8(15)7(14)4-10(12)17/h1-5H |
|---|
| InChI Key | InChIKey=WHPVYXDFIXRKLN-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as bromodiphenyl ethers. Bromodiphenyl ethers are compounds that contain two benzene groups linked to each other via an ether bond, and where at least one ring is substituted with a bromo group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Diphenylethers |
|---|
| Direct Parent | Bromodiphenyl ethers |
|---|
| Alternative Parents | |
|---|
| Substituents | - Bromodiphenyl ether
- Diaryl ether
- Phenoxy compound
- Phenol ether
- Halobenzene
- Bromobenzene
- Aryl halide
- Aryl bromide
- Ether
- Organic oxygen compound
- Hydrocarbon derivative
- Organooxygen compound
- Organobromide
- Organohalogen compound
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Liquid |
|---|
| Appearance | White crystalline solid. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | -5°C | | Boiling Point | Not Available | | Solubility | 9e-10 mg/mL at 20°C | | LogP | 6.84 |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-02t9-0333940000-42dd8a3ed64cf167100b | 2017-09-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0000090000-809056b74a1ce70d7d44 | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-0000090000-7f10ee2524d5c970e373 | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03di-0100190000-14eb8c2cc4adb8070e88 | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0000090000-d8c93920a7764fd1ef92 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-0000090000-ebc328172790629347c2 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0zi0-0129870000-a7109e0d4d1b31b6f938 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0000090000-fe5c3ca47b88f93a58cf | 2021-09-22 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-0000090000-fe5c3ca47b88f93a58cf | 2021-09-22 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0v00-5029000000-50aada19d00650a28d0d | 2021-09-22 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0000090000-a36382b147d8b58ad72d | 2021-09-22 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-0000090000-a36382b147d8b58ad72d | 2021-09-22 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0nmi-0009850000-e3839609dbbe3d0ab1aa | 2021-09-22 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Oral (4) ; inhalation (4) ; dermal (4) |
|---|
| Mechanism of Toxicity | Like other halogenated aromatic hydrocarbons, polybrominated diphenyl ethers bind to the cellular aryl hydrocarbon receptor (AhR), which regulates the synthesis of a variety of proteins. Activation of the AhR induces a number of enzymes, including cytochrome P-450-dependent monooxygenases of the CYP1A and CYP2B families, UDP-glucuronosyltransferase, and ethoxyresorufin-o-deethylase. PBDEs are also believed to disrupt the production, transport, and disposition of thyroid hormones. One mechanism of this involves metabolites ot PDBEs competing with thyroxine to bind to transthyretin, decreasing serum thyroid hormone levels. This change in thyroid hormone levels has been linked to both thyroid toxicity and neurobehavioral alterations. Certain PDBEs and their metabolites are also endocrine disruptors and may act as agonists at the estrogen receptors or antagonists at the androgen and progesterone receptors. (4, 1) |
|---|
| Metabolism | Polybrominated biphenyls can be absorbed through oral, inhalation, and dermal routes. Once in the body they distribute throughout and bioaccumulate in the blood, breast milk, and adipose tissue. The extent of PBDE metabolism depends on the degree of bromination. Metabolism is believed to involve debromination and methylation, resulting in phenolic metabolites. Metabolized and unmetabolized PDBE compounds are excreted mainly in the faeces. (4, 6) |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | Polybrominated diphenyl ethers (PBDEs) are flame-retardant man-made chemicals found in plastics used in a variety of consumer products to make them difficult to burn. Pentabromodiphenyl ethers are most commonly used as a flame retardant in flexible polyurethane foam. (4, 5) |
|---|
| Minimum Risk Level | Intermediate Inhalation: 0.006 mg/m3 (3)
Acute Oral: 0.03 mg/kg/day (3)
Intermediate Oral: 0.007 mg/kg/day (3) |
|---|
| Health Effects | Polybrominated diphenyl ethers may affect the thyroid gland and liver. Animals studies have also shown that PDBEs can cause neurobehavioral alterations and affect the immune system. (4) |
|---|
| Symptoms | Not Available |
|---|
| Treatment | EYES: irrigate opened eyes for several minutes under running water.
INGESTION: do not induce vomiting. Rinse mouth with water (never give anything by mouth to an unconscious person). Seek immediate medical advice.
SKIN: should be treated immediately by rinsing the affected parts in cold running water for at least 15 minutes, followed by thorough washing with soap and water. If necessary, the person should shower and change contaminated clothing and shoes, and then must seek medical attention.
INHALATION: supply fresh air. If required provide artificial respiration. |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB37516 |
|---|
| PubChem Compound ID | 36159 |
|---|
| ChEMBL ID | Not Available |
|---|
| ChemSpider ID | 33255 |
|---|
| KEGG ID | C18203 |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | C086401 |
|---|
| Stitch ID | 2,2',4,4',5-Pentabromobiphenyl Ether |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | 4495 |
|---|
| Wikipedia Link | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | - Hamers T, Kamstra JH, Sonneveld E, Murk AJ, Kester MH, Andersson PL, Legler J, Brouwer A: In vitro profiling of the endocrine-disrupting potency of brominated flame retardants. Toxicol Sci. 2006 Jul;92(1):157-73. Epub 2006 Apr 6. [16601080 ]
- Yannai, Shmuel. (2004) Dictionary of food compounds with CD-ROM: Additives, flavors, and ingredients. Boca Raton: Chapman & Hall/CRC.
- ATSDR - Agency for Toxic Substances and Disease Registry (2001). Minimal Risk Levels (MRLs) for Hazardous Substances. U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). [Link]
- ATSDR - Agency for Toxic Substances and Disease Registry (2004). Toxicological profile for polybrominated biphenyls and polybrominated diphenyl ethers (PBBs and PBDEs). U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). [Link]
- Wikipedia. Octabromodiphenyl ether. Last Updated 13 May 2009. [Link]
- Wikipedia. Polybrominated diphenyl ether. Last Updated 19 June 2009. [Link]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | | Gene | Gene Symbol | Gene ID | Interaction | Chromosome | Details |
|---|
|
|---|
| Down-Regulated Genes | | Gene | Gene Symbol | Gene ID | Interaction | Chromosome | Details |
|---|
|
|---|