| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2009-07-21 20:26:21 UTC |
|---|
| Update Date | 2014-12-24 20:25:50 UTC |
|---|
| Accession Number | T3D2721 |
|---|
| Identification |
|---|
| Common Name | Butabarbital |
|---|
| Class | Small Molecule |
|---|
| Description | Butabarbital (trade name Butisol) is a prescription barbiturate sleep aid. Butabarbital has a particularly fast onset of effects and short duration of action compared to other barbiturates, which makes it useful for certain applications such as treating severe insomnia and relieving anxiety before surgical procedures; however it is also relatively dangerous particularly when combined with alcohol, and so is now rarely used, although it is still prescribed in some Eastern European and South American countries. Its short duration of action gives butabarbital a high abuse potential, comparable to secobarbital. |
|---|
| Compound Type | - Amide
- Amine
- Anti-Anxiety Agent
- Barbiturate
- Drug
- Hypnotic and Sedative
- Metabolite
- Organic Compound
- Synthetic Compound
|
|---|
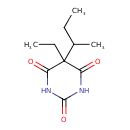
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | 5-Ethyl-5-(1-methylpropyl)-2,4,6(1H,3H,5H)-pyrimidinetrione | | 5-Ethyl-5-(1-methylpropyl)barbituric acid | | 5-Sec-butyl-5-ethyl-2,4,6(1H,3H,5H)-pyrimidinetrione | | 5-Sec-butyl-5-ethylbarbituric acid | | 5-Sec-butyl-5-ethylpyrimidine-2,4,6(1H,3H,5H)-trione | | Butabarbitone | | Butalan | | Butisol | | Butrate | | Sarisol No. 2 | | Secbubarbital | | Secbutabarbital | | Secbutobarbital | | Secbutobarbitone | | Sodium Butabarbital |
|
|---|
| Chemical Formula | C10H16N2O3 |
|---|
| Average Molecular Mass | 212.246 g/mol |
|---|
| Monoisotopic Mass | 212.116 g/mol |
|---|
| CAS Registry Number | 125-40-6 |
|---|
| IUPAC Name | 5-(butan-2-yl)-5-ethyl-1,3-diazinane-2,4,6-trione |
|---|
| Traditional Name | butabarbital |
|---|
| SMILES | CCC(C)C1(CC)C(O)=NC(=O)N=C1O |
|---|
| InChI Identifier | InChI=1/C10H16N2O3/c1-4-6(3)10(5-2)7(13)11-9(15)12-8(10)14/h6H,4-5H2,1-3H3,(H2,11,12,13,14,15) |
|---|
| InChI Key | InChIKey=ZRIHAIZYIMGOAB-UHFFFAOYNA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as barbituric acid derivatives. Barbituric acid derivatives are compounds containing a perhydropyrimidine ring substituted at C-2, -4 and -6 by oxo groups. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Diazines |
|---|
| Sub Class | Pyrimidines and pyrimidine derivatives |
|---|
| Direct Parent | Barbituric acid derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Barbiturate
- N-acyl urea
- Ureide
- 1,3-diazinane
- Dicarboximide
- Urea
- Carbonic acid derivative
- Carboxylic acid derivative
- Azacycle
- Organic nitrogen compound
- Organonitrogen compound
- Organooxygen compound
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Carbonyl group
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | 166.5°C | | Boiling Point | Not Available | | Solubility | 1360 mg/L | | LogP | 1.65 |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0a7i-8900000000-fc5f17ec8979e31b77ac | 2017-09-01 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-1290000000-73748d3c96bdcbc7692d | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0007-3900000000-c62b6d06318be5ee7a26 | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-9000000000-d815b39070a4a2b620ee | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0296-8950000000-e8d3b0ea461e5ad69068 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-9500000000-43d75b9170cae3f239fa | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000x-9400000000-ec7399384bd48aaa6ee4 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0900000000-121a04bb64c32af4a00f | 2021-09-23 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-3900000000-58caeb294d4571a30cf4 | 2021-09-23 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0002-9300000000-527f2d60d78505d84953 | 2021-09-23 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-1290000000-994157d74b9e27e50e2d | 2021-09-24 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-9000000000-90726b17dc36e29c5299 | 2021-09-24 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9100000000-85bbea89298999fca7c3 | 2021-09-24 | View Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-0a4l-6900000000-6e0cbc3d26c5541ad8eb | 2014-09-20 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Butabarbital binds at a distinct binding site associated with a Cl- ionopore at the GABAA receptor, increasing the duration of time for which the Cl- ionopore is open. The post-synaptic inhibitory effect of GABA in the thalamus is, therefore, prolonged. All of these effects are associated with marked decreases in GABA-sensitive neuronal calcium conductance (gCa). The net result of barbiturate action is acute potentiation of inhibitory GABAergic tone. Barbiturates also act through potent (if less well characterized) and direct inhibition of excitatory AMPA-type glutamate receptors, resulting in a profound suppression of glutamatergic neurotransmission. |
|---|
| Metabolism |
Route of Elimination: Barbiturates are metabolized primarily by the hepatic microsomal enzyme system, and most metabolic products are excreted in the urine. |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | Useful for certain applications such as treating severe insomnia and relieving anxiety before surgical procedures; |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | They cause slurred speech, disorientation and "drunken" behavior. They are physically and psychologically addictive. They cause slurred speech, disorientation and "drunken" behavior. They are physically and psychologically addictive. |
|---|
| Symptoms | Signs of overdose include confusion (severe), decrease in or loss of reflexes, drowsiness (severe), fever, irritability (continuing), low body temperature, poor judgment, shortness of breath or slow or troubled breathing, slow heartbeat, slurred speech, staggering, trouble in sleeping, unusual movements of the eyes, weakness (severe). |
|---|
| Treatment | Not Available |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB00237 |
|---|
| HMDB ID | HMDB14382 |
|---|
| PubChem Compound ID | 2479 |
|---|
| ChEMBL ID | CHEMBL449 |
|---|
| ChemSpider ID | 2385 |
|---|
| KEGG ID | C07827 |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | 3228 |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Butabarbital |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Butabarbital |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | T3D2721.pdf |
|---|
| General References | - Drugs.com [Link]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|