| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2009-07-21 20:26:56 UTC |
|---|
| Update Date | 2014-12-24 20:25:51 UTC |
|---|
| Accession Number | T3D2796 |
|---|
| Identification |
|---|
| Common Name | Loratadine |
|---|
| Class | Small Molecule |
|---|
| Description | Loratadine is a tricyclic antihistamine, which has a selective and peripheral H1-antagonist action. It has a long-lasting effect and does not normally cause drowsiness because it does not readily enter the central nervous system; An antiviral that is used in the prophylactic or symptomatic treatment of influenza A. It is also used as an antiparkinsonian agent, to treat extrapyramidal reactions, and for postherpetic neuralgia. The mechanisms of its effects in movement disorders are not well understood but probably reflect an increase in synthesis and release of dopamine, with perhaps some inhibition of dopamine uptake; Loratadine is a drug used to treat allergies. It is marketed by Schering-Plough under several trade names such as Claritin, Clarityn or Claratyne depending on the market, by Lek as Lomilan and by Wyeth as Alavert. It is also available as a generic; Loratadine is a drug used to treat allergies. It is marketed by Schering-Plough under several trade names such as Claritin, Clarityn or Claratyne depending on the market, by Lek as Lomilan and by Wyeth as Alavert. It is also available as a generic. Its active metabolite, desloratadine, is also on the market, though loratadine itself is the only drug of its class available over the counter (at least in the U.S. as of 2005. Loratadine is available off the shelf in the UK. |
|---|
| Compound Type | - Amine
- Anti-Allergic Agent
- Antipruritic
- Drug
- Ester
- Ether
- Food Toxin
- Histamine Antagonist
- Histamine H1 Antagonist, Non-Sedating
- Metabolite
- Organic Compound
- Organochloride
- Synthetic Compound
|
|---|
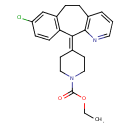
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | Aerotina | | Alavert | | Amantadine | | Anhissen | | Biloina | | Bonalerg | | Civeran | | Claritin | | Claritine | | Clarityn | | Clarityne | | Cronopen | | Flonidan | | Fristamin | | Histaloran | | Klaritin | | Lertamine | | Lisino | | Lomilan | | Loracert | | Loradex | | Loranox | | Lorastine | | Loratadina | | Loratadine antihistamine | | Loratadinum | | Loratidine | | Loratyne | | Loritine | | Nularef | | Restamine | | Roletra | | Sensibit |
|
|---|
| Chemical Formula | C22H23ClN2O2 |
|---|
| Average Molecular Mass | 382.883 g/mol |
|---|
| Monoisotopic Mass | 382.145 g/mol |
|---|
| CAS Registry Number | 79794-75-5 |
|---|
| IUPAC Name | ethyl 4-{13-chloro-4-azatricyclo[9.4.0.0^{3,8}]pentadeca-1(11),3(8),4,6,12,14-hexaen-2-ylidene}piperidine-1-carboxylate |
|---|
| Traditional Name | ethyl 4-{13-chloro-4-azatricyclo[9.4.0.0^{3,8}]pentadeca-1(11),3(8),4,6,12,14-hexaen-2-ylidene}piperidine-1-carboxylate |
|---|
| SMILES | CCOC(=O)N1CCC(CC1)=C1C2=CC=C(Cl)C=C2CCC2=C1N=CC=C2 |
|---|
| InChI Identifier | InChI=1S/C22H23ClN2O2/c1-2-27-22(26)25-12-9-15(10-13-25)20-19-8-7-18(23)14-17(19)6-5-16-4-3-11-24-21(16)20/h3-4,7-8,11,14H,2,5-6,9-10,12-13H2,1H3 |
|---|
| InChI Key | InChIKey=JCCNYMKQOSZNPW-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as benzocycloheptapyridines. These are aromatic compounds containing a benzene ring and a pyridine ring fused to a seven membered carbocycle. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Benzocycloheptapyridines |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Benzocycloheptapyridines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Benzocycloheptapyridine
- Piperidinecarboxylic acid
- Aryl chloride
- Aryl halide
- Piperidine
- Pyridine
- Benzenoid
- Heteroaromatic compound
- Carbamic acid ester
- Carbonic acid derivative
- Azacycle
- Hydrocarbon derivative
- Organic oxide
- Organooxygen compound
- Organonitrogen compound
- Organochloride
- Organohalogen compound
- Carbonyl group
- Organopnictogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | - Brain
- Central Nervous System
- Skin
|
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | 134-136°C | | Boiling Point | Not Available | | Solubility | 0.000011 mg/ml | | LogP | 5.2 |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-004i-6059000000-3d596b5ab5499c4089ca | 2017-09-01 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-qTof , Positive | splash10-001r-0039000000-928ed9c6b46ba83ebe20 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-001r-1279000000-1c522c39e53b6bbfb9aa | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-001r-0039000000-928ed9c6b46ba83ebe20 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-067r-1292000000-3869907bd01f927b6f22 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 75V, Positive | splash10-067i-0090000000-9bcbcadeda0bf07da702 | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 90V, Positive | splash10-0frx-0090000000-2f8b7b5acca254e4cb20 | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 40V, Positive | splash10-067i-0090000000-b247e61957d933538341 | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0009000000-dce8adc02434b224d589 | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0009000000-7d2b8c3f5f87f99fcf7a | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 60V, Positive | splash10-0api-0090000000-8419cf3b0c888e52c95b | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 45V, Positive | splash10-067r-0093000000-956a3618f6003ae45808 | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 40V, Positive | splash10-067i-0090000000-b62a29b4d6252b26f2bf | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 20V, Positive | splash10-001r-0009000000-d32917132c3359e65572 | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 20V, Positive | splash10-0019-0019000000-1acbfc6c05c2d600a966 | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 15V, Positive | splash10-001i-0009000000-4558440f48e82bf6eefb | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 30V, Positive | splash10-0019-0009000000-ea1b61e9df959d6680c2 | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 75V, Positive | splash10-067i-0090000000-cf7d75b43a3c2c0f0d1e | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 50V, Positive | splash10-066r-0090000000-c5d1377bb7159732d4db | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 40V, Positive | splash10-015i-0092000000-9bb7dcefe2f2f4f14bfd | 2021-09-20 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0009000000-41dd9857a0206fa0f4e8 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-06ri-1029000000-0c5a179194107a35af3e | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-015c-2091000000-fb50d1834a9a1d8d6c90 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001r-1009000000-78af7865fc3439c125c5 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-2009000000-df88de530c8ff41c69f4 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0adl-7069000000-a5804bf835988dc83d2a | 2016-08-03 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 600 MHz, CD3OD, experimental) | Not Available | 2012-12-05 | View Spectrum | | 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, H2O, experimental) | Not Available | 2012-12-05 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Rapidly absorbed following oral administration (40% bioavailability) |
|---|
| Mechanism of Toxicity | Loratadine competes with free histamine and exhibits specific, selective peripheral H1 antagonistic activity. This blocks the action of endogenous histamine, which subsequently leads to temporary relief of the negative symptoms (eg. nasal congestion, watery eyes) brought on by histamine. Loratadine has low affinity for cholinergic receptors and does not exhibit any appreciable alpha-adrenergic blocking activity in-vitro. Loratadine also appears to suppress the release of histamine and leukotrienes from animal mast cells, and the release of leukotrienes from human lung fragments, although the clinical importance of this is unknown. |
|---|
| Metabolism | Hepatic
Half Life: 8.4 hours |
|---|
| Toxicity Values | LD50=mg/kg (orally in rat) |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | Used to treat allergies. A self-medication that is used alone or in combination with pseudoephedrine sulfate for the symptomatic relief of seasonal allergic rhinitis. Also used for the symptomatic relief of pruritus, erythema, and urticaria associated with chronic idiopathic urticaria in patients (not for children under 6 unless directed by a clincian). |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Loratadine causes sedation and psychomotor impairment. |
|---|
| Symptoms | Somnolence, tachycardia, and headache. Psychomotor impairment, and antimuscarinic effects such as urinary retention, dry mouth, blurred vision, and gastrointestinal disturbances are the most common side effects. |
|---|
| Treatment | Treatment of overdosage would reasonably consist of emesis (ipecac syrup), except in patients with impaired consciousness, followed by the administration of activated charcoal to absorb any remaining drug. If vomiting is unsuccessful, or contraindicated, gastric lavage should be performed with normal saline. Saline cathartics may also be of value for rapid dilution of bowel contents. Loratadine is not eliminated by hemodialysis. It is not known if loratadine is eliminated by peritoneal dialysis. (15) |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB00455 |
|---|
| HMDB ID | HMDB05000 |
|---|
| PubChem Compound ID | 3957 |
|---|
| ChEMBL ID | CHEMBL998 |
|---|
| ChemSpider ID | 3820 |
|---|
| KEGG ID | C06818 |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | 210803 |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Loratadine |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Loratadine |
|---|
| References |
|---|
| Synthesis Reference | Alberto Stampa, Pelayo Camps, Gloria Rodriguez, Jordi Bosch, Maria del Carmen Onrubia, “Process for the preparation of loratadine.” U.S. Patent US6084100, issued July 04, 2000. |
|---|
| MSDS | Link |
|---|
| General References | - See S: Desloratadine for allergic rhinitis. Am Fam Physician. 2003 Nov 15;68(10):2015-6. [14655812 ]
- Menardo JL, Horak F, Danzig MR, Czarlewski W: A review of loratadine in the treatment of patients with allergic bronchial asthma. Clin Ther. 1997 Nov-Dec;19(6):1278-93; discussion 1523-4. [9444440 ]
- Howarth PH: Histamine and asthma: an appraisal based on specific H1-receptor antagonism. Clin Exp Allergy. 1990 Aug;20 Suppl 2:31-41. [1977506 ]
- Baroody FM, Naclerio RM: Antiallergic effects of H1-receptor antagonists. Allergy. 2000;55 Suppl 64:17-27. [11291777 ]
- Green LB, Hornyak JE, Hurvitz EA: Amantadine in pediatric patients with traumatic brain injury: a retrospective, case-controlled study. Am J Phys Med Rehabil. 2004 Dec;83(12):893-7. [15624567 ]
- Purohit A, Melac M, Pauli G, Frossard N: Comparative activity of cetirizine and desloratadine on histamine-induced wheal-and-flare responses during 24 hours. Ann Allergy Asthma Immunol. 2004 Jun;92(6):635-40. [15237765 ]
- Kornhuber J, Quack G, Danysz W, Jellinger K, Danielczyk W, Gsell W, Riederer P: Therapeutic brain concentration of the NMDA receptor antagonist amantadine. Neuropharmacology. 1995 Jul;34(7):713-21. [8532138 ]
- Deep P, Dagher A, Sadikot A, Gjedde A, Cumming P: Stimulation of dopa decarboxylase activity in striatum of healthy human brain secondary to NMDA receptor antagonism with a low dose of amantadine. Synapse. 1999 Dec 15;34(4):313-8. [10529725 ]
- Strong DK, Eisenstat DD, Bryson SM, Sitar DS, Arbus GS: Amantadine neurotoxicity in a pediatric patient with renal insufficiency. DICP. 1991 Nov;25(11):1175-7. [1763530 ]
- Ramboer I, Bumtbacea R, Lazarescu D, Radu JR: Cetirizine and loratadine: a comparison using the ED50 in skin reactions. J Int Med Res. 2000 Mar-Apr;28(2):69-77. [10898119 ]
- Simons FE, Silver NA, Gu X, Simons KJ: Clinical pharmacology of H1-antihistamines in the skin. J Allergy Clin Immunol. 2002 Nov;110(5):777-83. [12417888 ]
- Shiller AD, Burke DT, Kim HJ, Calvanio R, Dechman KG, Santini C: Treatment with amantadine potentiated motor learning in a patient with traumatic brain injury of 15 years' duration. Brain Inj. 1999 Sep;13(9):715-21. [10507453 ]
- Wilkinson R, Meythaler JM, Guin-Renfroe S: Neuroleptic malignant syndrome induced by haloperidol following traumatic brain injury. Brain Inj. 1999 Dec;13(12):1025-31. [10628507 ]
- Drugs.com [Link]
- RxList: The Internet Drug Index (2009). [Link]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | | Gene | Gene Symbol | Gene ID | Interaction | Chromosome | Details |
|---|
|
|---|
| Down-Regulated Genes | | Gene | Gene Symbol | Gene ID | Interaction | Chromosome | Details |
|---|
|
|---|