| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2009-07-21 20:27:07 UTC |
|---|
| Update Date | 2014-12-24 20:25:51 UTC |
|---|
| Accession Number | T3D2820 |
|---|
| Identification |
|---|
| Common Name | Pyridostigmine |
|---|
| Class | Small Molecule |
|---|
| Description | Pyridostigmine is only found in individuals that have used or taken this drug. It is a cholinesterase inhibitor with a slightly longer duration of action than neostigmine. It is used in the treatment of myasthenia gravis and to reverse the actions of muscle relaxants. Pyridostigmine inhibits acetylcholinesterase in the synaptic cleft by competing with acetylcholine for attachment to acetylcholinesterase, thus slowing down the hydrolysis of acetylcholine, and thereby increases efficiency of cholinergic transmission in the neuromuscular junction and prolonges the effects of acetylcholine. |
|---|
| Compound Type | - Amine
- Antimyasthenic Agent
- Cholinesterase Inhibitor
- Drug
- Ester
- Metabolite
- Organic Compound
- Parasympathomimetic
- Synthetic Compound
|
|---|
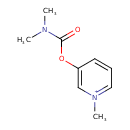
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | Amiasten | | Amygra | | Antilon | | Astinon | | Bromure de Pyridostigmine | | Bromuro de piridostigmina | | Kalymin | | Kalymin forte | | Kalymin N | | Kalymin retard | | Meshanon60 | | Mestinon | | Mestinon retard | | Mestinon-SR | | Myestin | | Pyridine N-Oxide | | Pyridostigmin bromid | | Pyridostigmine Bromide | | Pyridostigmine Bromine | | Pyridostigmini Bromidum | | Pyridostigminum | | Pyrimine | | Regonol |
|
|---|
| Chemical Formula | C9H13N2O2 |
|---|
| Average Molecular Mass | 181.211 g/mol |
|---|
| Monoisotopic Mass | 181.097 g/mol |
|---|
| CAS Registry Number | 155-97-5 |
|---|
| IUPAC Name | 3-[(dimethylcarbamoyl)oxy]-1-methylpyridin-1-ium |
|---|
| Traditional Name | pyridostigmine |
|---|
| SMILES | CN(C)C(=O)OC1=C[N+](C)=CC=C1 |
|---|
| InChI Identifier | InChI=1S/C9H13N2O2/c1-10(2)9(12)13-8-5-4-6-11(3)7-8/h4-7H,1-3H3/q+1 |
|---|
| InChI Key | InChIKey=RVOLLAQWKVFTGE-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as n-methylpyridinium compounds. These are methylpyridines that carry a methyl group at the 1-position. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Pyridines and derivatives |
|---|
| Sub Class | Methylpyridines |
|---|
| Direct Parent | N-methylpyridinium compounds |
|---|
| Alternative Parents | |
|---|
| Substituents | - N-methylpyridinium
- Pyridinium
- Carbamic acid ester
- Heteroaromatic compound
- Carbonic acid derivative
- Azacycle
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Organooxygen compound
- Organonitrogen compound
- Organic nitrogen compound
- Carbonyl group
- Hydrocarbon derivative
- Organic cation
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | 153°C | | Boiling Point | Not Available | | Solubility | 1.04e+00 g/L | | LogP | 1.554 |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0006-9700000000-144f11d8d4fca931e26f | 2017-09-01 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-4900000000-505a3902a67894832786 | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001j-7900000000-f2fb1dbcea23a916a60d | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0005-9100000000-5851c472c0f98c9da49e | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-1900000000-4020266c0445cc9e4d81 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00lr-9700000000-76c2110cb7085d434fd7 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-007c-9000000000-bf72b6b775960258441e | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0900000000-7a79dd4b3f240ec6c301 | 2021-09-22 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03ec-3900000000-f5675587ab409b8f06de | 2021-09-22 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9000000000-d30ae42d6c1e66ffefe8 | 2021-09-22 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | 2021-09-16 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Poorly absorbed from the GI tract with an oral bioavailability of 7.6 +/- 2.4%. |
|---|
| Mechanism of Toxicity | Pyridostigmine is a cholinesterase or acetylcholinesterase (AChE) inhibitor. A cholinesterase inhibitor (or 'anticholinesterase') suppresses the action of acetylcholinesterase. Because of its essential function, chemicals that interfere with the action of acetylcholinesterase are potent neurotoxins, causing excessive salivation and eye-watering in low doses, followed by muscle spasms and ultimately death. Nerve gases and many substances used in insecticides have been shown to act by binding a serine in the active site of acetylcholine esterase, inhibiting the enzyme completely. Acetylcholine esterase breaks down the neurotransmitter acetylcholine, which is released at nerve and muscle junctions, in order to allow the muscle or organ to relax. The result of acetylcholine esterase inhibition is that acetylcholine builds up and continues to act so that any nerve impulses are continually transmitted and muscle contractions do not stop. Among the most common acetylcholinesterase inhibitors are phosphorus-based compounds, which are designed to bind to the active site of the enzyme. The structural requirements are a phosphorus atom bearing two lipophilic groups, a leaving group (such as a halide or thiocyanate), and a terminal oxygen. |
|---|
| Metabolism | Hydrolysis by cholinesterases and by liver.
Half Life: 3 hours following oral administration. |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | For the treatment of myasthenia gravis. |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Acute exposure to cholinesterase inhibitors can cause a cholinergic crisis characterized by severe nausea/vomiting, salivation, sweating, bradycardia, hypotension, collapse, and convulsions. Increasing muscle weakness is a possibility and may result in death if respiratory muscles are involved. Accumulation of ACh at motor nerves causes overstimulation of nicotinic expression at the neuromuscular junction. When this occurs symptoms such as muscle weakness, fatigue, muscle cramps, fasciculation, and paralysis can be seen. When there is an accumulation of ACh at autonomic ganglia this causes overstimulation of nicotinic expression in the sympathetic system. Symptoms associated with this are hypertension, and hypoglycemia. Overstimulation of nicotinic acetylcholine receptors in the central nervous system, due to accumulation of ACh, results in anxiety, headache, convulsions, ataxia, depression of respiration and circulation, tremor, general weakness, and potentially coma. When there is expression of muscarinic overstimulation due to excess acetylcholine at muscarinic acetylcholine receptors symptoms of visual disturbances, tightness in chest, wheezing due to bronchoconstriction, increased bronchial secretions, increased salivation, lacrimation, sweating, peristalsis, and urination can occur. Certain reproductive effects in fertility, growth, and development for males and females have been linked specifically to organophosphate pesticide exposure. Most of the research on reproductive effects has been conducted on farmers working with pesticides and insecticdes in rural areas. In females menstrual cycle disturbances, longer pregnancies, spontaneous abortions, stillbirths, and some developmental effects in offspring have been linked to organophosphate pesticide exposure. Prenatal exposure has been linked to impaired fetal growth and development. Neurotoxic effects have also been linked to poisoning with OP pesticides causing four neurotoxic effects in humans: cholinergic syndrome, intermediate syndrome, organophosphate-induced delayed polyneuropathy (OPIDP), and chronic organophosphate-induced neuropsychiatric disorder (COPIND). These syndromes result after acute and chronic exposure to OP pesticides. |
|---|
| Symptoms | Symptoms of low dose exposure include excessive salivation and eye-watering. Acute dose symptoms include severe nausea/vomiting, salivation, sweating, bradycardia, hypotension, collapse, and convulsions. Increasing muscle weakness is a possibility and may result in death if respiratory muscles are involved. Hypertension, hypoglycemia, anxiety, headache, tremor and ataxia may also result. |
|---|
| Treatment | If the compound has been ingested, rapid gastric lavage should be performed using 5% sodium bicarbonate. For skin contact, the skin should be washed with soap and water. If the compound has entered the eyes, they should be washed with large quantities of isotonic saline or water. In serious cases, atropine and/or pralidoxime should be administered. Anti-cholinergic drugs work to counteract the effects of excess acetylcholine and reactivate AChE. Atropine can be used as an antidote in conjunction with pralidoxime or other pyridinium oximes (such as trimedoxime or obidoxime), though the use of '-oximes' has been found to be of no benefit, or possibly harmful, in at least two meta-analyses. Atropine is a muscarinic antagonist, and thus blocks the action of acetylcholine peripherally. |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB00545 |
|---|
| HMDB ID | HMDB14685 |
|---|
| PubChem Compound ID | 4991 |
|---|
| ChEMBL ID | CHEMBL1115 |
|---|
| ChemSpider ID | 4817 |
|---|
| KEGG ID | C07410 |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | 257330 |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Pyridostigmine |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Pyridostigmine |
|---|
| References |
|---|
| Synthesis Reference | Thomas Zich, “Preparation of substituted pyridine N-oxide compounds.” U.S. Patent US20040063957, issued April 01, 2004. |
|---|
| MSDS | Link |
|---|
| General References | - Singer W, Opfer-Gehrking TL, McPhee BR, Hilz MJ, Bharucha AE, Low PA: Acetylcholinesterase inhibition: a novel approach in the treatment of neurogenic orthostatic hypotension. J Neurol Neurosurg Psychiatry. 2003 Sep;74(9):1294-8. [12933939 ]
- Drugs.com [Link]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|