Selegiline (T3D2965)
| Record Information | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 2.0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Creation Date | 2009-07-21 20:28:13 UTC | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 2014-12-24 20:25:54 UTC | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Accession Number | T3D2965 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Common Name | Selegiline | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Small Molecule | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | A selective, irreversible inhibitor of Type B monoamine oxidase. It is used in newly diagnosed patients with Parkinson's disease. It may slow progression of the clinical disease and delay the requirement for levodopa therapy. It also may be given with levodopa upon onset of disability. (From AMA Drug Evaluations Annual, 1994, p385) The compound without isomeric designation is Deprenyl. [PubChem] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Compound Type |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
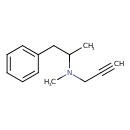
| Chemical Structure | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula | C13H17N | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Mass | 187.281 g/mol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Mass | 187.136 g/mol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS Registry Number | 14611-51-9 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name | methyl(1-phenylpropan-2-yl)(prop-2-yn-1-yl)amine | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional Name | D-deprenyl | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES | CC(CC1=CC=CC=C1)N(C)CC#C | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Identifier | InChI=1/C13H17N/c1-4-10-14(3)12(2)11-13-8-6-5-7-9-13/h1,5-9,12H,10-11H2,2-3H3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key | InChIKey=MEZLKOACVSPNER-UHFFFAOYNA-N | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | belongs to the class of organic compounds known as amphetamines and derivatives. These are organic compounds containing or derived from 1-phenylpropan-2-amine. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Benzenoids | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Benzene and substituted derivatives | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Phenethylamines | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Amphetamines and derivatives | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aromatic homomonocyclic compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Status | Detected and Not Quantified | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Origin | Exogenous | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biofluid Locations | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Tissue Locations | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Applications | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Roles | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Roles | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State | Solid | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | White powder. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Toxicity Profile | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Route of Exposure | Oral. Rapidly absorbed from the gastrointestinal tract. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mechanism of Toxicity | Although the mechanisms for selegiline's beneficial action in the treatment of Parkinson's disease are not fully understood, the selective, irreversible inhibition of monoamine oxidase type B (MAO-B) is thought to be of primary importance. MAO-B is involved in the oxidative deamination of dopamine in the brain. Selegiline binds to MAO-B within the nigrostriatal pathways in the central nervous system, thus blocking microsomal metabolism of dopamine and enhancing the dopaminergic activity in the substantial nigra. Selegiline may also increase dopaminergic activity through mechanisms other than inhibition of MAO-B. At higher doses, selegiline can also inhibit monozmine oxidase type A (MAO-A), allowing it to be used for the treatment of depression. MAO's activity is inhibited when selegiline binds to the isoalloxazine flavin adenine dinucleotide (FAD) at its active center | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolism | Half Life: 1.2-2 hours | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Toxicity Values | LD50: 63 mg/kg (Intravenous, Rat) (2) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Lethal Dose | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Uses/Sources | Monotherapy for initial treatment of Parkinson's disease, as well as an adjunct therapy in patients with a decreased response to levodopa/carbadopa. Also used for the palliative treatment of mild to moderate Alzheimer's disease and at higher doses, for the treatment of depression. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Minimum Risk Level | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Health Effects | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symptoms | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Treatment | Treatment of overdose with non-selective MAOIs is symptomatic and supportive. Induction of emesis or gastric lavage with instillation of charcoal slurry may be helpful in early poisoning, provided the airway has been protected against aspiration. Signs and symptoms of central nervous system stimulation, including convulsions, should be treated with diazepam, given slowly intravenously. Phenothiazine derivatives and central nervous system stimulants should be avoided. Hypotension and vascular collapse should be treated with intravenous fluids and, if necessary, blood pressure titration with an intravenous infusion of a dilute pressor agent. It should be noted that adrenergic agents may produce a markedly increased pressor response. Respiration should be supported by appropriate measures, including management of the airway, use of supplemental oxygen, and mechanical ventilatory assistance, as required. Body temperature should be monitored closely. Intensive management of hyperpyrexia may be required. Maintenance of fluid and electrolyte balance is essential. (9) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Normal Concentrations | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Abnormal Concentrations | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| DrugBank ID | DB01037 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| HMDB ID | HMDB15171 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PubChem Compound ID | 5195 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ChEMBL ID | CHEMBL972 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ChemSpider ID | 5007 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| KEGG ID | C07245 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| UniProt ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| OMIM ID | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ChEBI ID | 50217 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BioCyc ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CTD ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Stitch ID | Selegiline | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PDB ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ACToR ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikipedia Link | Selegiline | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference | Silvia Ott-Dembrowski, Richard Cyrus, Jorg Schmidt, Hans Waiblinger, “Preparation of selegiline.” U.S. Patent US5847216, issued March, 1962. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MSDS | Link | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| General References |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Gene Regulation | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Up-Regulated Genes | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Down-Regulated Genes | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Targets
- General Function:
- Primary amine oxidase activity
- Specific Function:
- Catalyzes the oxidative deamination of biogenic and xenobiotic amines and has important functions in the metabolism of neuroactive and vasoactive amines in the central nervous system and peripheral tissues. MAOB preferentially degrades benzylamine and phenylethylamine.
- Gene Name:
- MAOB
- Uniprot ID:
- P27338
- Molecular Weight:
- 58762.475 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | 0.091 uM | Not Available | BindingDB 15579 |
| Inhibitory | 0.97 uM | Not Available | BindingDB 15579 |
| Inhibitory | 1.96 uM | Not Available | BindingDB 15579 |
| IC50 | 0.000017 uM | Not Available | BindingDB 15579 |
| IC50 | 0.007 uM | Not Available | BindingDB 15579 |
| IC50 | 0.013 uM | Not Available | BindingDB 15579 |
| IC50 | 0.0148 uM | Not Available | BindingDB 15579 |
| IC50 | 0.017 uM | Not Available | BindingDB 15579 |
| IC50 | 0.0185 uM | Not Available | BindingDB 15579 |
| IC50 | 0.019 uM | Not Available | BindingDB 15579 |
| IC50 | 0.0196 uM | Not Available | BindingDB 15579 |
| IC50 | 0.01995 uM | Not Available | BindingDB 15579 |
| IC50 | 0.02 uM | Not Available | BindingDB 15579 |
| IC50 | 0.045 uM | Not Available | BindingDB 15579 |
| IC50 | 0.056 uM | Not Available | BindingDB 15579 |
| IC50 | 0.079 uM | Not Available | BindingDB 15579 |
| IC50 | 0.0852 uM | Not Available | BindingDB 15579 |
| IC50 | 0.334 uM | Not Available | BindingDB 15579 |
| IC50 | 19.6 uM | Not Available | BindingDB 15579 |
References
- Chen X, Ji ZL, Chen YZ: TTD: Therapeutic Target Database. Nucleic Acids Res. 2002 Jan 1;30(1):412-5. [11752352 ]
- Hayashi S, Nakata E, Morita A, Mizuno K, Yamamura K, Kato A, Ohashi K: Discovery of {1-[4-(2-{hexahydropyrrolo[3,4-c]pyrrol-2(1H)-yl}-1H-benzimidazol-1-yl)piperidin- 1-yl]cyclooctyl}methanol, systemically potent novel non-peptide agonist of nociceptin/orphanin FQ receptor as analgesic for the treatment of neuropathic pain: design, synthesis, and structure-activity relationships. Bioorg Med Chem. 2010 Nov 1;18(21):7675-99. doi: 10.1016/j.bmc.2010.07.034. Epub 2010 Jul 21. [20875743 ]
- Cavalli A, Bolognesi ML, Minarini A, Rosini M, Tumiatti V, Recanatini M, Melchiorre C: Multi-target-directed ligands to combat neurodegenerative diseases. J Med Chem. 2008 Feb 14;51(3):347-72. doi: 10.1021/jm7009364. Epub 2008 Jan 9. [18181565 ]
- Nag S, Lehmann L, Kettschau G, Heinrich T, Thiele A, Varrone A, Gulyas B, Halldin C: Synthesis and evaluation of [(1)(8)F]fluororasagiline, a novel positron emission tomography (PET) radioligand for monoamine oxidase B (MAO-B). Bioorg Med Chem. 2012 May 1;20(9):3065-71. doi: 10.1016/j.bmc.2012.02.056. Epub 2012 Mar 3. [22436387 ]
- Delogu G, Picciau C, Ferino G, Quezada E, Podda G, Uriarte E, Vina D: Synthesis, human monoamine oxidase inhibitory activity and molecular docking studies of 3-heteroarylcoumarin derivatives. Eur J Med Chem. 2011 Apr;46(4):1147-52. doi: 10.1016/j.ejmech.2011.01.033. Epub 2011 Jan 28. [21316817 ]
- Alcaro S, Gaspar A, Ortuso F, Milhazes N, Orallo F, Uriarte E, Yanez M, Borges F: Chromone-2- and -3-carboxylic acids inhibit differently monoamine oxidases A and B. Bioorg Med Chem Lett. 2010 May 1;20(9):2709-12. doi: 10.1016/j.bmcl.2010.03.081. Epub 2010 Mar 27. [20382016 ]
- Gaspar A, Reis J, Fonseca A, Milhazes N, Vina D, Uriarte E, Borges F: Chromone 3-phenylcarboxamides as potent and selective MAO-B inhibitors. Bioorg Med Chem Lett. 2011 Jan 15;21(2):707-9. doi: 10.1016/j.bmcl.2010.11.128. Epub 2010 Dec 5. [21194943 ]
- Gaspar A, Silva T, Yanez M, Vina D, Orallo F, Ortuso F, Uriarte E, Alcaro S, Borges F: Chromone, a privileged scaffold for the development of monoamine oxidase inhibitors. J Med Chem. 2011 Jul 28;54(14):5165-73. doi: 10.1021/jm2004267. Epub 2011 Jul 1. [21696156 ]
- Serra S, Ferino G, Matos MJ, Vazquez-Rodriguez S, Delogu G, Vina D, Cadoni E, Santana L, Uriarte E: Hydroxycoumarins as selective MAO-B inhibitors. Bioorg Med Chem Lett. 2012 Jan 1;22(1):258-61. doi: 10.1016/j.bmcl.2011.11.020. Epub 2011 Nov 11. [22137786 ]
- Huang L, Lu C, Sun Y, Mao F, Luo Z, Su T, Jiang H, Shan W, Li X: Multitarget-directed benzylideneindanone derivatives: anti-beta-amyloid (Abeta) aggregation, antioxidant, metal chelation, and monoamine oxidase B (MAO-B) inhibition properties against Alzheimer's disease. J Med Chem. 2012 Oct 11;55(19):8483-92. doi: 10.1021/jm300978h. Epub 2012 Oct 1. [22978824 ]
- Chimenti F, Fioravanti R, Bolasco A, Chimenti P, Secci D, Rossi F, Yanez M, Orallo F, Ortuso F, Alcaro S, Cirilli R, Ferretti R, Sanna ML: A new series of flavones, thioflavones, and flavanones as selective monoamine oxidase-B inhibitors. Bioorg Med Chem. 2010 Feb;18(3):1273-9. doi: 10.1016/j.bmc.2009.12.029. Epub 2010 Jan 4. [20045650 ]
- Distinto S, Yanez M, Alcaro S, Cardia MC, Gaspari M, Sanna ML, Meleddu R, Ortuso F, Kirchmair J, Markt P, Bolasco A, Wolber G, Secci D, Maccioni E: Synthesis and biological assessment of novel 2-thiazolylhydrazones and computational analysis of their recognition by monoamine oxidase B. Eur J Med Chem. 2012 Feb;48:284-95. doi: 10.1016/j.ejmech.2011.12.027. Epub 2011 Dec 24. [22222137 ]
- Chimenti F, Maccioni E, Secci D, Bolasco A, Chimenti P, Granese A, Carradori S, Alcaro S, Ortuso F, Yanez M, Orallo F, Cirilli R, Ferretti R, La Torre F: Synthesis, stereochemical identification, and selective inhibitory activity against human monoamine oxidase-B of 2-methylcyclohexylidene-(4-arylthiazol-2-yl)hydrazones. J Med Chem. 2008 Aug 28;51(16):4874-80. doi: 10.1021/jm800132g. Epub 2008 Jul 31. [18666768 ]
- Santana L, Gonzalez-Diaz H, Quezada E, Uriarte E, Yanez M, Vina D, Orallo F: Quantitative structure-activity relationship and complex network approach to monoamine oxidase A and B inhibitors. J Med Chem. 2008 Nov 13;51(21):6740-51. doi: 10.1021/jm800656v. Epub 2008 Oct 4. [18834112 ]
- Matos MJ, Vina D, Quezada E, Picciau C, Delogu G, Orallo F, Santana L, Uriarte E: A new series of 3-phenylcoumarins as potent and selective MAO-B inhibitors. Bioorg Med Chem Lett. 2009 Jun 15;19(12):3268-70. doi: 10.1016/j.bmcl.2009.04.085. Epub 2009 Apr 24. [19423346 ]
- Maccioni E, Alcaro S, Orallo F, Cardia MC, Distinto S, Costa G, Yanez M, Sanna ML, Vigo S, Meleddu R, Secci D: Synthesis of new 3-aryl-4,5-dihydropyrazole-1-carbothioamide derivatives. An investigation on their ability to inhibit monoamine oxidase. Eur J Med Chem. 2010 Oct;45(10):4490-8. doi: 10.1016/j.ejmech.2010.07.009. Epub 2010 Jul 17. [20702005 ]
- Desideri N, Bolasco A, Fioravanti R, Monaco LP, Orallo F, Yanez M, Ortuso F, Alcaro S: Homoisoflavonoids: natural scaffolds with potent and selective monoamine oxidase-B inhibition properties. J Med Chem. 2011 Apr 14;54(7):2155-64. doi: 10.1021/jm1013709. Epub 2011 Mar 15. [21405131 ]
- Matos MJ, Vazquez-Rodriguez S, Uriarte E, Santana L, Vina D: MAO inhibitory activity modulation: 3-Phenylcoumarins versus 3-benzoylcoumarins. Bioorg Med Chem Lett. 2011 Jul 15;21(14):4224-7. doi: 10.1016/j.bmcl.2011.05.074. Epub 2011 May 30. [21684743 ]
- Secci D, Carradori S, Bolasco A, Chimenti P, Yanez M, Ortuso F, Alcaro S: Synthesis and selective human monoamine oxidase inhibition of 3-carbonyl, 3-acyl, and 3-carboxyhydrazido coumarin derivatives. Eur J Med Chem. 2011 Oct;46(10):4846-52. doi: 10.1016/j.ejmech.2011.07.017. Epub 2011 Jul 19. [21872365 ]
- Matos MJ, Teran C, Perez-Castillo Y, Uriarte E, Santana L, Vina D: Synthesis and study of a series of 3-arylcoumarins as potent and selective monoamine oxidase B inhibitors. J Med Chem. 2011 Oct 27;54(20):7127-37. doi: 10.1021/jm200716y. Epub 2011 Sep 29. [21923181 ]
- Secci D, Bolasco A, Carradori S, D'Ascenzio M, Nescatelli R, Yanez M: Recent advances in the development of selective human MAO-B inhibitors: (hetero)arylidene-(4-substituted-thiazol-2-yl)hydrazines. Eur J Med Chem. 2012 Dec;58:405-17. doi: 10.1016/j.ejmech.2012.10.032. Epub 2012 Oct 26. [23153812 ]
- Fioravanti R, Bolasco A, Manna F, Rossi F, Orallo F, Yanez M, Vitali A, Ortuso F, Alcaro S: Synthesis and molecular modelling studies of prenylated pyrazolines as MAO-B inhibitors. Bioorg Med Chem Lett. 2010 Nov 15;20(22):6479-82. doi: 10.1016/j.bmcl.2010.09.061. Epub 2010 Sep 17. [20934874 ]
- Chimenti F, Secci D, Bolasco A, Chimenti P, Granese A, Carradori S, Maccioni E, Cardia MC, Yanez M, Orallo F, Alcaro S, Ortuso F, Cirilli R, Ferretti R, Distinto S, Kirchmair J, Langer T: Synthesis, semipreparative HPLC separation, biological evaluation, and 3D-QSAR of hydrazothiazole derivatives as human monoamine oxidase B inhibitors. Bioorg Med Chem. 2010 Jul 15;18(14):5063-70. doi: 10.1016/j.bmc.2010.05.070. Epub 2010 Jun 1. [20579890 ]
- Chimenti F, Secci D, Bolasco A, Chimenti P, Granese A, Carradori S, Yanez M, Orallo F, Sanna ML, Gallinella B, Cirilli R: Synthesis, stereochemical separation, and biological evaluation of selective inhibitors of human MAO-B: 1-(4-arylthiazol-2-yl)-2-(3-methylcyclohexylidene)hydrazines. J Med Chem. 2010 Sep 9;53(17):6516-20. doi: 10.1021/jm100120s. [20715818 ]
- Desideri N, Fioravanti R, Proietti Monaco L, Biava M, Yanez M, Ortuso F, Alcaro S: 1,5-Diphenylpenta-2,4-dien-1-ones as potent and selective monoamine oxidase-B inhibitors. Eur J Med Chem. 2013 Jan;59:91-100. doi: 10.1016/j.ejmech.2012.11.006. Epub 2012 Nov 15. [23207410 ]
- Lewellyn K, Bialonska D, Chaurasiya ND, Tekwani BL, Zjawiony JK: Synthesis and evaluation of aplysinopsin analogs as inhibitors of human monoamine oxidase A and B. Bioorg Med Chem Lett. 2012 Aug 1;22(15):4926-9. doi: 10.1016/j.bmcl.2012.06.058. Epub 2012 Jun 23. [22781190 ]
- Nag S, Kettschau G, Heinrich T, Varrone A, Lehmann L, Gulyas B, Thiele A, Keller E, Halldin C: Synthesis and biological evaluation of novel propargyl amines as potential fluorine-18 labeled radioligands for detection of MAO-B activity. Bioorg Med Chem. 2013 Jan 1;21(1):186-95. doi: 10.1016/j.bmc.2012.10.050. Epub 2012 Nov 15. [23211968 ]
- Van der Walt MM, Terre'Blanche G, Lourens AC, Petzer A, Petzer JP: Sulfanylphthalonitrile analogues as selective and potent inhibitors of monoamine oxidase B. Bioorg Med Chem Lett. 2012 Dec 15;22(24):7367-70. doi: 10.1016/j.bmcl.2012.10.070. Epub 2012 Oct 22. [23122857 ]
- Nag S, Lehmann L, Heinrich T, Thiele A, Kettschau G, Nakao R, Gulyas B, Halldin C: Synthesis of three novel fluorine-18 labeled analogues of L-deprenyl for positron emission tomography (PET) studies of monoamine oxidase B (MAO-B). J Med Chem. 2011 Oct 27;54(20):7023-9. doi: 10.1021/jm200710b. Epub 2011 Sep 29. [21923198 ]
- Rivara S, Piersanti G, Bartoccini F, Diamantini G, Pala D, Riccioni T, Stasi MA, Cabri W, Borsini F, Mor M, Tarzia G, Minetti P: Synthesis of (E)-8-(3-chlorostyryl)caffeine analogues leading to 9-deazaxanthine derivatives as dual A(2A) antagonists/MAO-B inhibitors. J Med Chem. 2013 Feb 14;56(3):1247-61. doi: 10.1021/jm301686s. Epub 2013 Jan 16. [23281824 ]
- Chimenti F, Carradori S, Secci D, Bolasco A, Bizzarri B, Chimenti P, Granese A, Yanez M, Orallo F: Synthesis and inhibitory activity against human monoamine oxidase of N1-thiocarbamoyl-3,5-di(hetero)aryl-4,5-dihydro-(1H)-pyrazole derivatives. Eur J Med Chem. 2010 Feb;45(2):800-4. doi: 10.1016/j.ejmech.2009.11.003. Epub 2009 Nov 6. [19926363 ]
- Chimenti F, Bolasco A, Secci D, Chimenti P, Granese A, Carradori S, Yanez M, Orallo F, Ortuso F, Alcaro S: Investigations on the 2-thiazolylhydrazyne scaffold: synthesis and molecular modeling of selective human monoamine oxidase inhibitors. Bioorg Med Chem. 2010 Aug 1;18(15):5715-23. doi: 10.1016/j.bmc.2010.06.007. Epub 2010 Jun 9. [20615716 ]
- Matos MJ, Vina D, Janeiro P, Borges F, Santana L, Uriarte E: New halogenated 3-phenylcoumarins as potent and selective MAO-B inhibitors. Bioorg Med Chem Lett. 2010 Sep 1;20(17):5157-60. doi: 10.1016/j.bmcl.2010.07.013. Epub 2010 Jul 8. [20659799 ]
- Jagrat M, Behera J, Yabanoglu S, Ercan A, Ucar G, Sinha BN, Sankaran V, Basu A, Jayaprakash V: Pyrazoline based MAO inhibitors: synthesis, biological evaluation and SAR studies. Bioorg Med Chem Lett. 2011 Jul 15;21(14):4296-300. doi: 10.1016/j.bmcl.2011.05.057. Epub 2011 May 25. [21680183 ]
- Di Santo R, Costi R, Roux A, Artico M, Befani O, Meninno T, Agostinelli E, Palmegiani P, Turini P, Cirilli R, Ferretti R, Gallinella B, La Torre F: Design, synthesis, and biological activities of pyrrolylethanoneamine derivatives, a novel class of monoamine oxidases inhibitors. J Med Chem. 2005 Jun 30;48(13):4220-3. [15974574 ]
- Toprakci M, Yelekci K: Docking studies on monoamine oxidase-B inhibitors: estimation of inhibition constants (K(i)) of a series of experimentally tested compounds. Bioorg Med Chem Lett. 2005 Oct 15;15(20):4438-46. [16137882 ]
- Mishra N, Sasmal D: Development of selective and reversible pyrazoline based MAO-B inhibitors: virtual screening, synthesis and biological evaluation. Bioorg Med Chem Lett. 2011 Apr 1;21(7):1969-73. doi: 10.1016/j.bmcl.2011.02.030. Epub 2011 Feb 13. [21377879 ]
- General Function:
- Serotonin binding
- Specific Function:
- Catalyzes the oxidative deamination of biogenic and xenobiotic amines and has important functions in the metabolism of neuroactive and vasoactive amines in the central nervous system and peripheral tissues. MAOA preferentially oxidizes biogenic amines such as 5-hydroxytryptamine (5-HT), norepinephrine and epinephrine.
- Gene Name:
- MAOA
- Uniprot ID:
- P21397
- Molecular Weight:
- 59681.27 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | 9.06 uM | Not Available | BindingDB 15579 |
| Inhibitory | 67.25 uM | Not Available | BindingDB 15579 |
| IC50 | 0.06725 uM | Not Available | BindingDB 15579 |
| IC50 | 1.2 uM | Not Available | BindingDB 15579 |
| IC50 | 67.25 uM | Not Available | BindingDB 15579 |
| IC50 | 67.6083 uM | Not Available | BindingDB 15579 |
| IC50 | 68.73 uM | Not Available | BindingDB 15579 |
| IC50 | 70.2 uM | Not Available | BindingDB 15579 |
References
- Patkar AA, Pae CU, Masand PS: Transdermal selegiline: the new generation of monoamine oxidase inhibitors. CNS Spectr. 2006 May;11(5):363-75. [16641841 ]
- Azzaro AJ, Ziemniak J, Kemper E, Campbell BJ, VanDenBerg C: Pharmacokinetics and absolute bioavailability of selegiline following treatment of healthy subjects with the selegiline transdermal system (6 mg/24 h): a comparison with oral selegiline capsules. J Clin Pharmacol. 2007 Oct;47(10):1256-67. Epub 2007 Aug 22. [17715422 ]
- Lee KC, Chen JJ: Transdermal selegiline for the treatment of major depressive disorder. Neuropsychiatr Dis Treat. 2007;3(5):527-37. [19300583 ]
- Baker GB, Sowa B, Todd KG: Amine oxidases and their inhibitors: what can they tell us about neuroprotection and the development of drugs for neuropsychiatric disorders? J Psychiatry Neurosci. 2007 Sep;32(5):313-5. [17823646 ]
- Secci D, Carradori S, Bolasco A, Chimenti P, Yanez M, Ortuso F, Alcaro S: Synthesis and selective human monoamine oxidase inhibition of 3-carbonyl, 3-acyl, and 3-carboxyhydrazido coumarin derivatives. Eur J Med Chem. 2011 Oct;46(10):4846-52. doi: 10.1016/j.ejmech.2011.07.017. Epub 2011 Jul 19. [21872365 ]
- Cavalli A, Bolognesi ML, Minarini A, Rosini M, Tumiatti V, Recanatini M, Melchiorre C: Multi-target-directed ligands to combat neurodegenerative diseases. J Med Chem. 2008 Feb 14;51(3):347-72. doi: 10.1021/jm7009364. Epub 2008 Jan 9. [18181565 ]
- Chimenti F, Maccioni E, Secci D, Bolasco A, Chimenti P, Granese A, Carradori S, Alcaro S, Ortuso F, Yanez M, Orallo F, Cirilli R, Ferretti R, La Torre F: Synthesis, stereochemical identification, and selective inhibitory activity against human monoamine oxidase-B of 2-methylcyclohexylidene-(4-arylthiazol-2-yl)hydrazones. J Med Chem. 2008 Aug 28;51(16):4874-80. doi: 10.1021/jm800132g. Epub 2008 Jul 31. [18666768 ]
- Santana L, Gonzalez-Diaz H, Quezada E, Uriarte E, Yanez M, Vina D, Orallo F: Quantitative structure-activity relationship and complex network approach to monoamine oxidase A and B inhibitors. J Med Chem. 2008 Nov 13;51(21):6740-51. doi: 10.1021/jm800656v. Epub 2008 Oct 4. [18834112 ]
- Chimenti F, Fioravanti R, Bolasco A, Chimenti P, Secci D, Rossi F, Yanez M, Orallo F, Ortuso F, Alcaro S: Chalcones: a valid scaffold for monoamine oxidases inhibitors. J Med Chem. 2009 May 14;52(9):2818-24. doi: 10.1021/jm801590u. [19378991 ]
- Matos MJ, Vina D, Quezada E, Picciau C, Delogu G, Orallo F, Santana L, Uriarte E: A new series of 3-phenylcoumarins as potent and selective MAO-B inhibitors. Bioorg Med Chem Lett. 2009 Jun 15;19(12):3268-70. doi: 10.1016/j.bmcl.2009.04.085. Epub 2009 Apr 24. [19423346 ]
- Matos MJ, Vina D, Picciau C, Orallo F, Santana L, Uriarte E: Synthesis and evaluation of 6-methyl-3-phenylcoumarins as potent and selective MAO-B inhibitors. Bioorg Med Chem Lett. 2009 Sep 1;19(17):5053-5. doi: 10.1016/j.bmcl.2009.07.039. Epub 2009 Jul 10. [19628387 ]
- Chimenti F, Carradori S, Secci D, Bolasco A, Bizzarri B, Chimenti P, Granese A, Yanez M, Orallo F: Synthesis and inhibitory activity against human monoamine oxidase of N1-thiocarbamoyl-3,5-di(hetero)aryl-4,5-dihydro-(1H)-pyrazole derivatives. Eur J Med Chem. 2010 Feb;45(2):800-4. doi: 10.1016/j.ejmech.2009.11.003. Epub 2009 Nov 6. [19926363 ]
- Chimenti F, Fioravanti R, Bolasco A, Chimenti P, Secci D, Rossi F, Yanez M, Orallo F, Ortuso F, Alcaro S, Cirilli R, Ferretti R, Sanna ML: A new series of flavones, thioflavones, and flavanones as selective monoamine oxidase-B inhibitors. Bioorg Med Chem. 2010 Feb;18(3):1273-9. doi: 10.1016/j.bmc.2009.12.029. Epub 2010 Jan 4. [20045650 ]
- Chimenti F, Secci D, Bolasco A, Chimenti P, Granese A, Carradori S, Maccioni E, Cardia MC, Yanez M, Orallo F, Alcaro S, Ortuso F, Cirilli R, Ferretti R, Distinto S, Kirchmair J, Langer T: Synthesis, semipreparative HPLC separation, biological evaluation, and 3D-QSAR of hydrazothiazole derivatives as human monoamine oxidase B inhibitors. Bioorg Med Chem. 2010 Jul 15;18(14):5063-70. doi: 10.1016/j.bmc.2010.05.070. Epub 2010 Jun 1. [20579890 ]
- Chimenti F, Bolasco A, Secci D, Chimenti P, Granese A, Carradori S, Yanez M, Orallo F, Ortuso F, Alcaro S: Investigations on the 2-thiazolylhydrazyne scaffold: synthesis and molecular modeling of selective human monoamine oxidase inhibitors. Bioorg Med Chem. 2010 Aug 1;18(15):5715-23. doi: 10.1016/j.bmc.2010.06.007. Epub 2010 Jun 9. [20615716 ]
- Matos MJ, Vina D, Janeiro P, Borges F, Santana L, Uriarte E: New halogenated 3-phenylcoumarins as potent and selective MAO-B inhibitors. Bioorg Med Chem Lett. 2010 Sep 1;20(17):5157-60. doi: 10.1016/j.bmcl.2010.07.013. Epub 2010 Jul 8. [20659799 ]
- Maccioni E, Alcaro S, Orallo F, Cardia MC, Distinto S, Costa G, Yanez M, Sanna ML, Vigo S, Meleddu R, Secci D: Synthesis of new 3-aryl-4,5-dihydropyrazole-1-carbothioamide derivatives. An investigation on their ability to inhibit monoamine oxidase. Eur J Med Chem. 2010 Oct;45(10):4490-8. doi: 10.1016/j.ejmech.2010.07.009. Epub 2010 Jul 17. [20702005 ]
- Chimenti F, Secci D, Bolasco A, Chimenti P, Granese A, Carradori S, Yanez M, Orallo F, Sanna ML, Gallinella B, Cirilli R: Synthesis, stereochemical separation, and biological evaluation of selective inhibitors of human MAO-B: 1-(4-arylthiazol-2-yl)-2-(3-methylcyclohexylidene)hydrazines. J Med Chem. 2010 Sep 9;53(17):6516-20. doi: 10.1021/jm100120s. [20715818 ]
- Delogu G, Picciau C, Ferino G, Quezada E, Podda G, Uriarte E, Vina D: Synthesis, human monoamine oxidase inhibitory activity and molecular docking studies of 3-heteroarylcoumarin derivatives. Eur J Med Chem. 2011 Apr;46(4):1147-52. doi: 10.1016/j.ejmech.2011.01.033. Epub 2011 Jan 28. [21316817 ]
- Desideri N, Bolasco A, Fioravanti R, Monaco LP, Orallo F, Yanez M, Ortuso F, Alcaro S: Homoisoflavonoids: natural scaffolds with potent and selective monoamine oxidase-B inhibition properties. J Med Chem. 2011 Apr 14;54(7):2155-64. doi: 10.1021/jm1013709. Epub 2011 Mar 15. [21405131 ]
- Matos MJ, Vazquez-Rodriguez S, Uriarte E, Santana L, Vina D: MAO inhibitory activity modulation: 3-Phenylcoumarins versus 3-benzoylcoumarins. Bioorg Med Chem Lett. 2011 Jul 15;21(14):4224-7. doi: 10.1016/j.bmcl.2011.05.074. Epub 2011 May 30. [21684743 ]
- Matos MJ, Teran C, Perez-Castillo Y, Uriarte E, Santana L, Vina D: Synthesis and study of a series of 3-arylcoumarins as potent and selective monoamine oxidase B inhibitors. J Med Chem. 2011 Oct 27;54(20):7127-37. doi: 10.1021/jm200716y. Epub 2011 Sep 29. [21923181 ]
- Distinto S, Yanez M, Alcaro S, Cardia MC, Gaspari M, Sanna ML, Meleddu R, Ortuso F, Kirchmair J, Markt P, Bolasco A, Wolber G, Secci D, Maccioni E: Synthesis and biological assessment of novel 2-thiazolylhydrazones and computational analysis of their recognition by monoamine oxidase B. Eur J Med Chem. 2012 Feb;48:284-95. doi: 10.1016/j.ejmech.2011.12.027. Epub 2011 Dec 24. [22222137 ]
- Secci D, Bolasco A, Carradori S, D'Ascenzio M, Nescatelli R, Yanez M: Recent advances in the development of selective human MAO-B inhibitors: (hetero)arylidene-(4-substituted-thiazol-2-yl)hydrazines. Eur J Med Chem. 2012 Dec;58:405-17. doi: 10.1016/j.ejmech.2012.10.032. Epub 2012 Oct 26. [23153812 ]
- Desideri N, Fioravanti R, Proietti Monaco L, Biava M, Yanez M, Ortuso F, Alcaro S: 1,5-Diphenylpenta-2,4-dien-1-ones as potent and selective monoamine oxidase-B inhibitors. Eur J Med Chem. 2013 Jan;59:91-100. doi: 10.1016/j.ejmech.2012.11.006. Epub 2012 Nov 15. [23207410 ]
- Fioravanti R, Bolasco A, Manna F, Rossi F, Orallo F, Yanez M, Vitali A, Ortuso F, Alcaro S: Synthesis and molecular modelling studies of prenylated pyrazolines as MAO-B inhibitors. Bioorg Med Chem Lett. 2010 Nov 15;20(22):6479-82. doi: 10.1016/j.bmcl.2010.09.061. Epub 2010 Sep 17. [20934874 ]
- Alcaro S, Gaspar A, Ortuso F, Milhazes N, Orallo F, Uriarte E, Yanez M, Borges F: Chromone-2- and -3-carboxylic acids inhibit differently monoamine oxidases A and B. Bioorg Med Chem Lett. 2010 May 1;20(9):2709-12. doi: 10.1016/j.bmcl.2010.03.081. Epub 2010 Mar 27. [20382016 ]
- Gaspar A, Silva T, Yanez M, Vina D, Orallo F, Ortuso F, Uriarte E, Alcaro S, Borges F: Chromone, a privileged scaffold for the development of monoamine oxidase inhibitors. J Med Chem. 2011 Jul 28;54(14):5165-73. doi: 10.1021/jm2004267. Epub 2011 Jul 1. [21696156 ]
- Serra S, Ferino G, Matos MJ, Vazquez-Rodriguez S, Delogu G, Vina D, Cadoni E, Santana L, Uriarte E: Hydroxycoumarins as selective MAO-B inhibitors. Bioorg Med Chem Lett. 2012 Jan 1;22(1):258-61. doi: 10.1016/j.bmcl.2011.11.020. Epub 2011 Nov 11. [22137786 ]
- Huang L, Lu C, Sun Y, Mao F, Luo Z, Su T, Jiang H, Shan W, Li X: Multitarget-directed benzylideneindanone derivatives: anti-beta-amyloid (Abeta) aggregation, antioxidant, metal chelation, and monoamine oxidase B (MAO-B) inhibition properties against Alzheimer's disease. J Med Chem. 2012 Oct 11;55(19):8483-92. doi: 10.1021/jm300978h. Epub 2012 Oct 1. [22978824 ]
- Jagrat M, Behera J, Yabanoglu S, Ercan A, Ucar G, Sinha BN, Sankaran V, Basu A, Jayaprakash V: Pyrazoline based MAO inhibitors: synthesis, biological evaluation and SAR studies. Bioorg Med Chem Lett. 2011 Jul 15;21(14):4296-300. doi: 10.1016/j.bmcl.2011.05.057. Epub 2011 May 25. [21680183 ]
- Mishra N, Sasmal D: Development of selective and reversible pyrazoline based MAO-B inhibitors: virtual screening, synthesis and biological evaluation. Bioorg Med Chem Lett. 2011 Apr 1;21(7):1969-73. doi: 10.1016/j.bmcl.2011.02.030. Epub 2011 Feb 13. [21377879 ]
- General Function:
- Serotonin receptor activity
- Specific Function:
- G-protein coupled receptor for 5-hydroxytryptamine (serotonin). Also functions as a receptor for various drugs and psychoactive substances. Ligand binding causes a conformation change that triggers signaling via guanine nucleotide-binding proteins (G proteins) and modulates the activity of down-stream effectors, such as adenylate cyclase. Beta-arrestin family members inhibit signaling via G proteins and mediate activation of alternative signaling pathways. Signaling inhibits adenylate cyclase activity and activates a phosphatidylinositol-calcium second messenger system that regulates the release of Ca(2+) ions from intracellular stores. Plays a role in the regulation of 5-hydroxytryptamine release and in the regulation of dopamine and 5-hydroxytryptamine metabolism. Plays a role in the regulation of dopamine and 5-hydroxytryptamine levels in the brain, and thereby affects neural activity, mood and behavior. Plays a role in the response to anxiogenic stimuli.
- Gene Name:
- HTR1A
- Uniprot ID:
- P08908
- Molecular Weight:
- 46106.335 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | >10 uM | Not Available | BindingDB 15579 |
References
- Toll L, Berzetei-Gurske IP, Polgar WE, Brandt SR, Adapa ID, Rodriguez L, Schwartz RW, Haggart D, O'Brien A, White A, Kennedy JM, Craymer K, Farrington L, Auh JS: Standard binding and functional assays related to medications development division testing for potential cocaine and opiate narcotic treatment medications. NIDA Res Monogr. 1998 Mar;178:440-66. [9686407 ]
- General Function:
- G-protein coupled amine receptor activity
- Specific Function:
- Dopamine receptor whose activity is mediated by G proteins which activate adenylyl cyclase.
- Gene Name:
- DRD1
- Uniprot ID:
- P21728
- Molecular Weight:
- 49292.765 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | >10 uM | Not Available | BindingDB 15579 |
References
- Toll L, Berzetei-Gurske IP, Polgar WE, Brandt SR, Adapa ID, Rodriguez L, Schwartz RW, Haggart D, O'Brien A, White A, Kennedy JM, Craymer K, Farrington L, Auh JS: Standard binding and functional assays related to medications development division testing for potential cocaine and opiate narcotic treatment medications. NIDA Res Monogr. 1998 Mar;178:440-66. [9686407 ]