| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2009-07-21 20:28:14 UTC |
|---|
| Update Date | 2014-12-24 20:25:54 UTC |
|---|
| Accession Number | T3D2966 |
|---|
| Identification |
|---|
| Common Name | Carphenazine |
|---|
| Class | Small Molecule |
|---|
| Description | Carphenazine is only found in individuals that have used or taken this drug. It is an antipsychotic drug, used in hospitalized patients in the management of chronic schizophrenic psychoses.A yellow, powdered, phenothiazine antipsychotic agent used in the treatment of acute or chronic schizophrenia. The term phenothiazines is used to describe the largest of the five main classes of neuroleptic antipsychotic drugs. These drugs have antipsychotic and, often, antiemetic properties, although they may also cause severe side effects such as akathisia, tardive dyskinesia and extrapyramidal symptoms. Carphenazine blocks postsynaptic mesolimbic dopaminergic D1 and D2 receptors in the brain; depresses the release of hypothalamic and hypophyseal hormones and is believed to depress the reticular activating system thus affecting basal metabolism, body temperature, wakefulness, vasomotor tone, and emesis. |
|---|
| Compound Type | - Amine
- Antipsychotic Agent
- Drug
- Ester
- Ether
- Metabolite
- Organic Compound
- Synthetic Compound
|
|---|
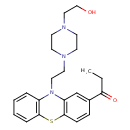
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | Carfenazine | | Carphenazin | | Procethazine | | Proketazin | | Proketazine |
|
|---|
| Chemical Formula | C23H29N3O2S |
|---|
| Average Molecular Mass | 411.560 g/mol |
|---|
| Monoisotopic Mass | 411.198 g/mol |
|---|
| CAS Registry Number | 2622-30-2 |
|---|
| IUPAC Name | 1-(10-{2-[4-(2-hydroxyethyl)piperazin-1-yl]ethyl}-10H-phenothiazin-2-yl)propan-1-one |
|---|
| Traditional Name | carfenazina |
|---|
| SMILES | CCC(=O)C1=CC2=C(SC3=CC=CC=C3N2CCN2CCN(CCO)CC2)C=C1 |
|---|
| InChI Identifier | InChI=1S/C23H29N3O2S/c1-2-21(28)18-7-8-23-20(17-18)26(19-5-3-4-6-22(19)29-23)14-13-24-9-11-25(12-10-24)15-16-27/h3-8,17,27H,2,9-16H2,1H3 |
|---|
| InChI Key | InChIKey=DYCNETKPCXPXNW-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as phenothiazines. These are polycyclic aromatic compounds containing a phenothiazine moiety, which is a linear tricyclic system that consists of a two benzene rings joined by a para-thiazine ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Benzothiazines |
|---|
| Sub Class | Phenothiazines |
|---|
| Direct Parent | Phenothiazines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Phenothiazine
- Alkyldiarylamine
- Diarylthioether
- Aryl thioether
- Tertiary aliphatic/aromatic amine
- Aryl alkyl ketone
- Aryl ketone
- N-alkylpiperazine
- Para-thiazine
- 1,4-diazinane
- Piperazine
- Benzenoid
- 1,2-aminoalcohol
- Ketone
- Tertiary amine
- Tertiary aliphatic amine
- Thioether
- Alkanolamine
- Azacycle
- Hydrocarbon derivative
- Primary alcohol
- Organic oxide
- Organopnictogen compound
- Organooxygen compound
- Organonitrogen compound
- Organic nitrogen compound
- Organic oxygen compound
- Amine
- Alcohol
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | |
|---|
| Biological Roles | |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | 175-177°C | | Boiling Point | Not Available | | Solubility | 9.05e-02 g/L | | LogP | 3.3 |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-003r-1249000000-8269c1aea27b4519caf5 | 2017-09-01 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-014i-4489800000-a2dc84d530af6c491e7e | 2017-10-06 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0326900000-6e5ae095bf0ef6084542 | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0bu0-2792000000-1cff5a929ddf220c741b | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0bu1-5932000000-98b40f4a2cdbae56d3ab | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0005900000-6bb5170e920dc2485681 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udm-0196100000-4cbb6b3fa5eccbfc7980 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0k97-9653000000-000631b921931d7be9ba | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0000900000-94a2b7e7b061d0929453 | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01q9-0190400000-27679b67f9c21cea78da | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-022a-8931000000-36b009ad5cd7ac7aec2b | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0000900000-474df7b273d6c5e26ef6 | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-0038900000-91fbc50a2f95f1bd7526 | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0udi-2192000000-96e0f1beaa722034f19a | 2021-10-11 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | A yellow, powdered, phenothiazine antipsychotic agent used in the treatment of acute or chronic schizophrenia. The term "phenothiazines" is used to describe the largest of the five main classes of neuroleptic antipsychotic drugs. These drugs have antipsychotic and, often, antiemetic properties, although they may also cause severe side effects such as akathisia, tardive dyskinesia and extrapyramidal symptoms. Carphenazine blocks postsynaptic mesolimbic dopaminergic D1 and D2 receptors in the brain; depresses the release of hypothalamic and hypophyseal hormones and is believed to depress the reticular activating system thus affecting basal metabolism, body temperature, wakefulness, vasomotor tone, and emesis. |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | Used in the treatment of acute or chronic schizophrenic reactions in hospitalized patients. |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB01038 |
|---|
| HMDB ID | HMDB15172 |
|---|
| PubChem Compound ID | 25137957 |
|---|
| ChEMBL ID | Not Available |
|---|
| ChemSpider ID | 21865853 |
|---|
| KEGG ID | Not Available |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | 51235 |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Carphenazine |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Tislow, R.F., Bruce, W.F. and Page, J.A.; US. Patent 3,023,146; February 27,1962; assigned to American Home Products Corporation.
Sherlock, M.H. and Sperber, N.; US. Patent 2,985,654; May 23, 1961; assigned to Schering
Corporation. |
|---|
| MSDS | Not Available |
|---|
| General References | - Wishart DS, Knox C, Guo AC, Cheng D, Shrivastava S, Tzur D, Gautam B, Hassanali M: DrugBank: a knowledgebase for drugs, drug actions and drug targets. Nucleic Acids Res. 2008 Jan;36(Database issue):D901-6. Epub 2007 Nov 29. [18048412 ]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|