| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2009-07-21 20:28:18 UTC |
|---|
| Update Date | 2014-12-24 20:25:54 UTC |
|---|
| Accession Number | T3D2976 |
|---|
| Identification |
|---|
| Common Name | Diphenhydramine |
|---|
| Class | Small Molecule |
|---|
| Description | Diphenhydramine is a histamine H1 antagonist used as an antiemetic, antitussive, for dermatoses and pruritus, for hypersensitivity reactions, as a hypnotic, an antiparkinson, and as an ingredient in common cold preparations. It has some undesired antimuscarinic and sedative effects. -- Pubchem; Pseudoephedrine is a phenethylamine, and an isomer of ephedrine. Pseudoephedrine is the International Nonproprietary Name (INN) of the (1S,2S)- diastereomer of ephedrine (which has 1R,2S- configuration). Other names are (+)-pseudoephedrine and D-pseudoephedrine (Reynolds, 1989). The enantiomer (-)-(1R,2R)-Pseudoephedrine has fewer side-effects, fewer central nervous system (CNS) stimulatory effects, does not reduce to d-methamphetamine, yet retains its efficacy as a decongestant. However, the patent holder for (-)-Pseudoephedrine (Pfizer/Warner-Lambert) has not yet sought or received government approval for its sale to the public.(US Patent 6,495,529); Treatment for urinary incontinence is an unlabeled use for these medications. Unlabeled use means doctors can use the medication to treat a condition other than that for which it was first approved by the U.S. Food and Drug Administration (FDA). These medications are approved by the FDA for the treatment of nasal congestion caused by colds or allergies. However it has also been successful in treating stress incontinence by increasing the pressure (tension) exerted by the muscles of the bladder neck and the urethra, which helps retain the urine within the bladder. Despite being one of the oldest antihistamines on the market, it is by and large the most effective antihistamine available, either by prescription or over-the-counter, and has been shown to exceed the effectiveness of even the latest prescription drugs. Consequently, it is frequently used when an allergic reaction requires fast, effective reversal of the (often dangerous) effects of a massive histamine release. However, it is not always the drug of choice for treating allergies. Like many other first generation antihistamines, is also a potent anticholinergic agent. This leads to profound drowsiness as a very common side-effect, along with the possibilities of motor impairment (ataxia), dry mouth and throat, flushed skin, rapid or irregular heartbeat (tachycardia), blurred vision at near point due to lack of accommodation (cycloplegia), abnormal sensitivity to bright light (photophobia), pupil dilatation, urinary retention, constipation, difficulty concentrating, short-term memory loss, visual disturbances, hallucinations, confusion, erectile dysfunction, and delirium. -- Wikipedia;. |
|---|
| Compound Type | - Amine
- Anesthetic
- Anesthetic, Local
- Anti-Allergic Agent
- Antidyskinetic
- Antiemetic
- Antiparkinson Agent
- Antipruritic
- Antitussive Agent
- Drug
- Ethanolamine Derivative
- Ether
- Food Toxin
- Histamine H1 Antagonist
- Hypnotic and Sedative
- Metabolite
- Organic Compound
- Synthetic Compound
|
|---|
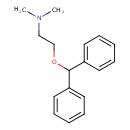
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | 2-(Benzhydryloxy)-N,N-dimethylethylamine | | 2-diphenylmethoxy-N,N-demthylethanamine | | Aleryl | | Alledryl | | Allerdryl | | Allergan | | Allergina | | alpha-(2-Dimethylaminoethoxy)diphenylmethane | | Banophen | | Baramine | | Beldin | | Belix | | Benadryl | | Bendylate | | Benylan | | Benzantin | | beta-Dimethylaminoethanol diphenylmethyl ether | | beta-Dimethylaminoethyl benzhydryl ether | | Dermodrin | | Desentol | | Dibondrin | | Difenhidramina | | Difenhydramine | | Dimedrol | | Dimedrolum | | Dimethylamine benzhydryl ester | | Diphamine | | Diphantine | | Diphen | | Diphenhist | | Diphenhydraminum | | Dobacen | | Dormarex 2 | | Genahist | | Histaxin | | Hyrexin | | N-(2-(Diphenylmethoxy)ethyl)-N,N-dimethylamine | | Nytol Quickcaps | | Nytol Quickgels | | O-benzhydryldimethylaminoethanol | | Restamin | | Siladryl | | Silphen Cough | | β-dimethylaminoethyl benzhydryl ether |
|
|---|
| Chemical Formula | C17H21NO |
|---|
| Average Molecular Mass | 255.355 g/mol |
|---|
| Monoisotopic Mass | 255.162 g/mol |
|---|
| CAS Registry Number | 58-73-1 |
|---|
| IUPAC Name | [2-(diphenylmethoxy)ethyl]dimethylamine |
|---|
| Traditional Name | diphenhydramine |
|---|
| SMILES | CN(C)CCOC(C1=CC=CC=C1)C1=CC=CC=C1 |
|---|
| InChI Identifier | InChI=1S/C17H21NO/c1-18(2)13-14-19-17(15-9-5-3-6-10-15)16-11-7-4-8-12-16/h3-12,17H,13-14H2,1-2H3 |
|---|
| InChI Key | InChIKey=ZZVUWRFHKOJYTH-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as diphenylmethanes. Diphenylmethanes are compounds containing a diphenylmethane moiety, which consists of a methane wherein two hydrogen atoms are replaced by two phenyl groups. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Diphenylmethanes |
|---|
| Direct Parent | Diphenylmethanes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Diphenylmethane
- Benzylether
- Tertiary aliphatic amine
- Tertiary amine
- Ether
- Dialkyl ether
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Amine
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | |
|---|
| Biological Roles | |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | 161-162°C | | Boiling Point | 150-165°C at 2.00E+00 mm Hg | | Solubility | 3060 mg/L (at 37°C) | | LogP | 3.27 |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0a4i-9100000000-7b9eb1f702c76f6b6062 | 2017-09-12 | View Spectrum | | GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0a4i-9100000000-7b9eb1f702c76f6b6062 | 2018-05-18 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-066r-6900000000-84b4a2c53eae448ca1bd | 2017-08-28 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, N/A (Annotated) | splash10-0006-0090000000-a71e531b49571f09ab25 | 2012-07-25 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, N/A (Annotated) | splash10-00kk-0980000000-cce91896f34562beeffa | 2012-07-25 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, N/A (Annotated) | splash10-001i-1900000000-4c29bd5e0eab94596eaa | 2012-07-25 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - EI-B (Unknown) , Positive | splash10-0a4i-9100000000-ab642e8f86de11af896c | 2012-08-31 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , negative | splash10-000t-0910000000-3576ca5ec77ab87354ec | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , positive | splash10-014i-0900000000-0ccd6de9ca58593fd490 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , positive | splash10-014i-0900000000-3568aae4c27ffe333c0e | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , positive | splash10-014i-0900000000-22e0c745355c836f1535 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , positive | splash10-014i-0900000000-6bb69a66a343440c64f8 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , positive | splash10-014i-0900000000-1b87d83415d22bb17f1b | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , positive | splash10-0gb9-0900000000-50251362d768cd1b1c4f | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , positive | splash10-0gb9-0900000000-9f37a733a05f5b01d313 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , positive | splash10-014i-0900000000-7d73b95ed09ea2d72260 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , positive | splash10-0gb9-2900000000-1666a8ab5a6d81c0bd01 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , positive | splash10-014i-0900000000-3f31841d858381760f03 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , positive | splash10-014i-0900000000-de872e048c7e12cef490 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , positive | splash10-014i-0900000000-de872e048c7e12cef490 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-014i-0900000000-789a4a3fe118d5bf2456 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-014i-2900000000-8ef94659f614b9ff24ca | 2017-09-14 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-1190000000-b33f7871c289d81b8648 | 2017-07-25 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0ab9-6690000000-814b7cb7faf094058c00 | 2017-07-25 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00xr-9500000000-98351e9c274a79fda2d0 | 2017-07-25 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-1290000000-3911d08f3498d2ef857f | 2017-07-26 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0ue9-4890000000-3d0fdfdee0fffe9f228f | 2017-07-26 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0059-9500000000-41b5f1e1a6122e43b285 | 2017-07-26 | View Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-0a4i-9200000000-b9014c8fc8ccbba24163 | 2014-09-20 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 600 MHz, H2O, experimental) | Not Available | 2012-12-05 | View Spectrum | | 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, H2O, experimental) | Not Available | 2012-12-05 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Oral; topical ; parenteral(intramuscular or intravenous injection). Quickly absorbed with maximum activity occurring in approximately one hour. |
|---|
| Mechanism of Toxicity | Diphenhydramine competes with free histamine for binding at HA-receptor sites. This antagonizes the effects of histamine on HA-receptors, leading to a reduction of the negative symptoms brought on by histamine HA-receptor binding. |
|---|
| Metabolism | Hepatic and renal
Route of Elimination: Little, if any, is excreted unchanged in the urine; most appears as the degradation products of metabolic transformation in the liver, which are almost completely excreted within 24 hours.
Half Life: 1-4 hours |
|---|
| Toxicity Values | LD50: 500 mg/kg (Oral, Rat) (2) |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | For the treatment of symptoms associated with Vertigo/Meniere's disease, nausea and vomiting, motion sickness and insect bite. |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | They cause slurred speech, disorientation and "drunken" behavior. They are physically and psychologically addictive. |
|---|
| Symptoms | Considerable overdosage can lead to myocardial infarction (heart attack), serious ventricular dysrhythmias, coma and death. |
|---|
| Treatment | There is no specific antidote for diphenhydramine toxicity, but the anticholinergic syndrome has been treated with physostigmine for severe delirium or tachycardia. Benzodiazepines may be administered to decrease the likelihood of psychosis, agitation, and seizures in patients who are prone to these symptoms. |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB01075 |
|---|
| HMDB ID | HMDB01927 |
|---|
| PubChem Compound ID | 3100 |
|---|
| ChEMBL ID | CHEMBL657 |
|---|
| ChemSpider ID | 2989 |
|---|
| KEGG ID | C06960 |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | 4636 |
|---|
| BioCyc ID | CPD-10890 |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Diphenylhydramine |
|---|
| PDB ID | 2PM |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Diphenhydramine |
|---|
| References |
|---|
| Synthesis Reference | Alison B. Lukacsko, Joseph J. Piala, “Effect of a combination of a terbutaline, diphenhydramine and ranitidine composition on gastrointestinal injury produced by nonsteroidal anti-inflammatory compositions.” U.S. Patent US5260333, issued December, 1983. |
|---|
| MSDS | Link |
|---|
| General References | - Raphael GD, Angello JT, Wu MM, Druce HM: Efficacy of diphenhydramine vs desloratadine and placebo in patients with moderate-to-severe seasonal allergic rhinitis. Ann Allergy Asthma Immunol. 2006 Apr;96(4):606-14. [16680933 ]
- Wishart DS, Knox C, Guo AC, Cheng D, Shrivastava S, Tzur D, Gautam B, Hassanali M: DrugBank: a knowledgebase for drugs, drug actions and drug targets. Nucleic Acids Res. 2008 Jan;36(Database issue):D901-6. Epub 2007 Nov 29. [18048412 ]
- Drugs.com [Link]
- Wikipedia. Diphenhydramine. Last updated on 10 October 2014. [Link]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|