| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2009-07-21 20:28:34 UTC |
|---|
| Update Date | 2014-12-24 20:25:55 UTC |
|---|
| Accession Number | T3D3012 |
|---|
| Identification |
|---|
| Common Name | Ciclopirox |
|---|
| Class | Small Molecule |
|---|
| Description | Ciclopirox is only found in individuals that have used or taken this drug. It is a synthetic antifungal agent for topical dermatologic use. [Wikipedia] Unlike antifungals such as itraconazole and terbinafine, which affect sterol synthesis, ciclopirox is thought to act through the chelation of polyvalent metal cations, such as Fe3+ and Al3+. These cations inhibit many enzymes, including cytochromes, thus disrupting cellular activities such as mitochondrial electron transport processes and energy production. Ciclopirox also appears to modify the plasma membrane of fungi, resulting in the disorganization of internal structures. The anti-inflammatory action of ciclopirox is most likely due to inhibition of 5-lipoxygenase and cyclooxygenase. Ciclopirox may exert its effect by disrupting DNA repair, cell division signals and structures (mitotic spindles) as well as some elements of intracellular transport. |
|---|
| Compound Type | - Antifungal Agent
- Drug
- Ester
- Metabolite
- Organic Compound
- Synthetic Compound
|
|---|
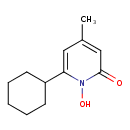
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | 6-Cyclohexyl-1-hydroxy-4-methyl-2(1H)-pyridinone | | Batrafen | | Ciclodan | | Ciclopirox Olamin | | Ciclopirox-Olamin | | Ciclopiroxolamine | | Ciclopiroxum | | CNL8 | | HOE 296b | | HOE-296b | | Loprox | | Mycoster | | Penlac | | Stieprox |
|
|---|
| Chemical Formula | C12H17NO2 |
|---|
| Average Molecular Mass | 207.269 g/mol |
|---|
| Monoisotopic Mass | 207.126 g/mol |
|---|
| CAS Registry Number | 29342-05-0 |
|---|
| IUPAC Name | 6-cyclohexyl-1-hydroxy-4-methyl-1,2-dihydropyridin-2-one |
|---|
| Traditional Name | penlac |
|---|
| SMILES | CC1=CC(=O)N(O)C(=C1)C1CCCCC1 |
|---|
| InChI Identifier | InChI=1S/C12H17NO2/c1-9-7-11(13(15)12(14)8-9)10-5-3-2-4-6-10/h7-8,10,15H,2-6H2,1H3 |
|---|
| InChI Key | InChIKey=SCKYRAXSEDYPSA-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as pyridinones. Pyridinones are compounds containing a pyridine ring, which bears a ketone. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Pyridines and derivatives |
|---|
| Sub Class | Hydropyridines |
|---|
| Direct Parent | Pyridinones |
|---|
| Alternative Parents | |
|---|
| Substituents | - Methylpyridine
- Pyridinone
- Dihydropyridine
- Heteroaromatic compound
- Lactam
- Azacycle
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | |
|---|
| Biological Roles | |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | 143°C | | Boiling Point | Not Available | | Solubility | 1.41e+00 g/L | | LogP | 2.3 |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-02vi-1900000000-b0302ddc025a747a3484 | 2017-09-01 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0390000000-1d9227c74bcc86583df3 | 2016-08-02 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-2690000000-963d9e37afedc3b4a600 | 2016-08-02 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05mo-9600000000-de9b944829cc25a5681b | 2016-08-02 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-3090000000-ac55f8f5496920dc9ca4 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-0490000000-6cd8e595360c6bddf33a | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a6r-5900000000-7869913422c2e64d2101 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0090000000-69c98b0c95a4f7a6ef58 | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-4490000000-d28507b6dbcfe8f98141 | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-066r-9700000000-4dafee1a9eed2bbabe7b | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0090000000-bc1578d935014f0ac50f | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-05is-6930000000-c7140620c24b41758ee7 | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0f6x-9400000000-8c3e2f33da6d3911ea34 | 2021-10-11 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Rapidly absorbed after oral administration. Mean absorption of ciclopirox after application to nails of all twenty digits and adjacent 5 millimeters of skin once daily for 6 months in patients with dermatophytic onychomycoses was less than 5% of the applied dose. Ciclopirox olamine also penetrates into hair and through the epidermis and hair follicles into sebaceous glands and dermis. |
|---|
| Mechanism of Toxicity | Unlike antifungals such as itraconazole and terbinafine, which affect sterol synthesis, ciclopirox is thought to act through the chelation of polyvalent metal cations, such as Fe3+ and Al3+. These cations inhibit many enzymes, including cytochromes, thus disrupting cellular activities such as mitochondrial electron transport processes and energy production. Ciclopirox also appears to modify the plasma membrane of fungi, resulting in the disorganization of internal structures. The anti-inflammatory action of ciclopirox is most likely due to inhibition of 5-lipoxygenase and cyclooxygenase.
ciclopirox may exert its effect by disrupting DNA repair, cell division signals and structures (mitotic spindles) as well as some elements of intracellular transport. |
|---|
| Metabolism | Glucuronidation is the main metabolic pathway of ciclopirox.
Route of Elimination: Most of the compound is excreted either unchanged or as glucuronide. After oral administration of 10 mg of radiolabeled drug (14C-ciclopirox) to healthy volunteers, approximately 96% of the radioactivity was excreted renally within 12 hours of administration. Ninety-four percent of the renally excreted radioactivity was in the form of glucuronides.
Half Life: 1.7 hours for 1% topical solution. |
|---|
| Toxicity Values | LD50: >10 ml/kg (Oral, Rat) (2) |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | Used as a topical treatment in immunocompetent patients with mild to moderate onychomycosis of fingernails and toenails without lunula involvement, due to Trichophyton rubrum. |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB01188 |
|---|
| HMDB ID | HMDB15319 |
|---|
| PubChem Compound ID | 2749 |
|---|
| ChEMBL ID | CHEMBL1413 |
|---|
| ChemSpider ID | 2647 |
|---|
| KEGG ID | Not Available |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | 453011 |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Ciclopirox |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Ciclopirox |
|---|
| References |
|---|
| Synthesis Reference | Lohaus, G.and Dittmar, W.; U.S. Patents 3,972,888; August 3, 1976; and 3,883,545; May 13, 1975; both assigned to Hoechst A .G. |
|---|
| MSDS | Link |
|---|
| General References | - Niewerth M, Kunze D, Seibold M, Schaller M, Korting HC, Hube B: Ciclopirox olamine treatment affects the expression pattern of Candida albicans genes encoding virulence factors, iron metabolism proteins, and drug resistance factors. Antimicrob Agents Chemother. 2003 Jun;47(6):1805-17. [12760852 ]
- Wishart DS, Knox C, Guo AC, Cheng D, Shrivastava S, Tzur D, Gautam B, Hassanali M: DrugBank: a knowledgebase for drugs, drug actions and drug targets. Nucleic Acids Res. 2008 Jan;36(Database issue):D901-6. Epub 2007 Nov 29. [18048412 ]
- Sigle HC, Thewes S, Niewerth M, Korting HC, Schafer-Korting M, Hube B: Oxygen accessibility and iron levels are critical factors for the antifungal action of ciclopirox against Candida albicans. J Antimicrob Chemother. 2005 May;55(5):663-73. Epub 2005 Mar 24. [15790671 ]
- Qadripur SA: [Antimycotic therapy. 2. Antimycotic chemotherapeutic agents: imidazole derivatives, tolciclate, haloprogin, ciclopiroxolamin]. Fortschr Med. 1983 Mar 10;101(9):355-63. [6303928 ]
- Beikert FC, Le MT, Koeninger A, Technau K, Clad A: Recurrent vulvovaginal candidosis: focus on the vulva. Mycoses. 2011 Nov;54(6):e807-10. doi: 10.1111/j.1439-0507.2011.02030.x. Epub 2011 May 25. [21615545 ]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | | Gene | Gene Symbol | Gene ID | Interaction | Chromosome | Details |
|---|
|
|---|