| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2009-07-21 20:28:43 UTC |
|---|
| Update Date | 2014-12-24 20:25:56 UTC |
|---|
| Accession Number | T3D3030 |
|---|
| Identification |
|---|
| Common Name | Bromodiphenhydramine |
|---|
| Class | Small Molecule |
|---|
| Description | Bromodiphenhydramine is only found in individuals that have used or taken this drug. It is an ethanolamine antihistamine with antimicrobial property. Bromodiphenhydramine is used in the control of cutaneous allergies. Ethanolamine antihistamines produce marked sedation in most patients. Bromodiphenhydramine competes with free histamine for binding at HA-receptor sites. This antagonizes the effects of histamine on HA-receptors, leading to a reduction of the negative symptoms brought on by histamine HA-receptor binding. |
|---|
| Compound Type | - Amine
- Bromide Compound
- Drug
- Ether
- Histamine Antagonist
- Metabolite
- Organic Compound
- Organobromide
- Synthetic Compound
|
|---|
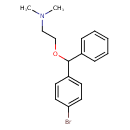
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | 2-(P-Bromo-alpha-phenylbenzyloxy)-N,N-dimethylethylamine | | Amodryl | | beta-(P-Bromobenzhydryloxy)ethyldimethylamine | | beta-Dimethylaminoethyl P-bromobenzhydryl ether | | Bromazin | | Bromazina | | Bromazine | | Bromazinum | | Bromdiphenhydramine |
|
|---|
| Chemical Formula | C17H20BrNO |
|---|
| Average Molecular Mass | 334.251 g/mol |
|---|
| Monoisotopic Mass | 333.073 g/mol |
|---|
| CAS Registry Number | 118-23-0 |
|---|
| IUPAC Name | {2-[(4-bromophenyl)(phenyl)methoxy]ethyl}dimethylamine |
|---|
| Traditional Name | bromodiphenhydramine |
|---|
| SMILES | CN(C)CCOC(C1=CC=CC=C1)C1=CC=C(Br)C=C1 |
|---|
| InChI Identifier | InChI=1/C17H20BrNO/c1-19(2)12-13-20-17(14-6-4-3-5-7-14)15-8-10-16(18)11-9-15/h3-11,17H,12-13H2,1-2H3 |
|---|
| InChI Key | InChIKey=NUNIWXHYABYXKF-UHFFFAOYNA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as diphenylmethanes. Diphenylmethanes are compounds containing a diphenylmethane moiety, which consists of a methane wherein two hydrogen atoms are replaced by two phenyl groups. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Diphenylmethanes |
|---|
| Direct Parent | Diphenylmethanes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Diphenylmethane
- Benzylether
- Bromobenzene
- Halobenzene
- Aryl bromide
- Aryl halide
- Tertiary aliphatic amine
- Tertiary amine
- Ether
- Dialkyl ether
- Organobromide
- Organohalogen compound
- Amine
- Hydrocarbon derivative
- Organopnictogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Organonitrogen compound
- Organooxygen compound
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | |
|---|
| Biological Roles | |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | 3.45e-03 g/L | | LogP | 4 |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0a4i-9000000000-53342d9a3abac3e5c130 | 2017-09-12 | View Spectrum | | GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0a4i-9000000000-53342d9a3abac3e5c130 | 2018-05-18 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0a4i-9170000000-a9ca360a39867bdc60e3 | 2017-09-01 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-1029000000-87dd03a792f708d9d560 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01q9-4298000000-6b6342526e6f4ecb97e7 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0ufr-7940000000-034e1b52c692b487913f | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0019000000-ef5c1d55ed2ce8849a56 | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0233-9272000000-29ea7428b9a497e3fd80 | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a6u-3490000000-1cd8fd6f5553771b70e7 | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-1019000000-1f077c44d5a8983889d9 | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-008c-8198000000-4408b93513107a5fa257 | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00dl-9230000000-62fdc401b129d1d31d65 | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000x-0094000000-3cf414b28e1dcbff7ea2 | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0006-0090000000-9617a3dfb8b832dc2bb3 | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-1090000000-cc1dcdb2d999990b6388 | 2021-10-11 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Well absorbed in the digestive tract. |
|---|
| Mechanism of Toxicity | Bromodiphenhydramine competes with free histamine for binding at HA-receptor sites. This antagonizes the effects of histamine on HA-receptors, leading to a reduction of the negative symptoms brought on by histamine HA-receptor binding. |
|---|
| Metabolism | Hepatic (cytochrome P-450 system); some renal.
Half Life: 1 to 4 hours |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | For management of symptoms related to hay fever and other types of allergy and used to help bring up phlegm, thin secretions, and make a cough productive. |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Signs of overdose include wheezing, tightness in the chest, fever, itching, bad cough, blue skin color, fits, swelling of face, lips, tongue, or throat. |
|---|
| Treatment | Not Available |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB01237 |
|---|
| HMDB ID | HMDB15367 |
|---|
| PubChem Compound ID | 2444 |
|---|
| ChEMBL ID | Not Available |
|---|
| ChemSpider ID | 2350 |
|---|
| KEGG ID | Not Available |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | 59177 |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Bromodiphenhydramine |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| References |
|---|
| Synthesis Reference | DrugSyn.org |
|---|
| MSDS | T3D3030.pdf |
|---|
| General References | - Drugs.com [Link]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|