| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2009-07-21 20:28:47 UTC |
|---|
| Update Date | 2014-12-24 20:25:56 UTC |
|---|
| Accession Number | T3D3039 |
|---|
| Identification |
|---|
| Common Name | Hexobarbital |
|---|
| Class | Small Molecule |
|---|
| Description | Hexobarbital is only found in individuals that have used or taken this drug. It is a barbiturate that is effective as a hypnotic and sedative. [PubChem]Hexobarbital binds at a distinct binding site associated with a Cl- ionopore at the GABA-A receptor, increasing the duration of time for which the Cl- ionopore is open. The post-synaptic inhibitory effect of GABA in the thalamus is, therefore, prolonged. |
|---|
| Compound Type | - Adjuvant
- Amide
- Amine
- Barbiturate
- Drug
- GABA Modulator
- Hypnotic and Sedative
- Metabolite
- Organic Compound
- Synthetic Compound
|
|---|
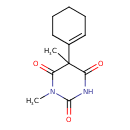
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | 5-(1-Cyclohexen-1-yl)-1,5-dimethyl-2,4,6(1H,3H,5H)-pyrimidinetrione | | 5-(1-Cyclohexen-1-yl)-1,5-dimethylbarbituric acid | | 5-Cyclohex-1-enyl-1,5-dimethyl-pyrimidine-2,4,6-trione | | Evipal | | Evipan | | Hexobarbitone | | Methexenyl | | Methylhexabital | | Tobinal |
|
|---|
| Chemical Formula | C12H16N2O3 |
|---|
| Average Molecular Mass | 236.267 g/mol |
|---|
| Monoisotopic Mass | 236.116 g/mol |
|---|
| CAS Registry Number | 56-29-1 |
|---|
| IUPAC Name | 5-(cyclohex-1-en-1-yl)-1,5-dimethyl-1,3-diazinane-2,4,6-trione |
|---|
| Traditional Name | hexobarbital |
|---|
| SMILES | CN1C(=O)N=C(O)C(C)(C2=CCCCC2)C1=O |
|---|
| InChI Identifier | InChI=1/C12H16N2O3/c1-12(8-6-4-3-5-7-8)9(15)13-11(17)14(2)10(12)16/h6H,3-5,7H2,1-2H3,(H,13,15,17) |
|---|
| InChI Key | InChIKey=UYXAWHWODHRRMR-UHFFFAOYNA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as pyrimidones. Pyrimidones are compounds that contain a pyrimidine ring, which bears a ketone. Pyrimidine is a 6-membered ring consisting of four carbon atoms and two nitrogen centers at the 1- and 3- ring positions. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Diazines |
|---|
| Sub Class | Pyrimidines and pyrimidine derivatives |
|---|
| Direct Parent | Pyrimidones |
|---|
| Alternative Parents | |
|---|
| Substituents | - Pyrimidone
- Hydropyrimidine
- 1,2,5,6-tetrahydropyrimidine
- Dicarboximide
- Carbonic acid derivative
- Azacycle
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Carboxylic acid derivative
- Organic oxide
- Hydrocarbon derivative
- Organic nitrogen compound
- Organooxygen compound
- Organonitrogen compound
- Carbonyl group
- Organopnictogen compound
- Organic oxygen compound
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | - Cytoplasm
- Extracellular
- Membrane
|
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | 146.5°C | | Boiling Point | Not Available | | Solubility | 435 mg/L (at 25°C) | | LogP | 1.98 |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-00gi-9420000000-001b8f1462933b4fb9c1 | 2017-09-12 | View Spectrum | | GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-00gi-9420000000-001b8f1462933b4fb9c1 | 2018-05-18 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-004i-3940000000-827e61b1deec4916f579 | 2017-09-01 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-1190000000-58f9b3cb0025cd8911aa | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-2920000000-e4d6ce12b6d33fff56d8 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0uxu-9100000000-55244be11c0d8667c413 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000f-7980000000-db4b09996693417c3e09 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0bt9-5900000000-3fac68a4cc2e19e26087 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9200000000-8495751984d2901a8826 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0390000000-d08c51477abaf85bbfa5 | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-1930000000-cc97a14668452a4e91e1 | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-6900000000-3951ea29d6dcc2dca21b | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-3490000000-509890f29192f5ebf006 | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4l-5970000000-fb452294b839815fbe4b | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000x-9450000000-ae3101e9d97123ad0a93 | 2021-10-11 | View Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-05cu-9310000000-eeec363075c613d35696 | 2014-09-20 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 90 MHz, CDCl3, experimental) | Not Available | 2014-09-20 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 25.16 MHz, CDCl3, experimental) | Not Available | 2014-09-23 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Oral |
|---|
| Mechanism of Toxicity | Hexobarbital binds at a distinct binding site associated with a Cl- ionopore at the GABA-A receptor, increasing the duration of time for which the Cl- ionopore is open. The post-synaptic inhibitory effect of GABA in the thalamus is, therefore, prolonged. |
|---|
| Metabolism | Hepatic. |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | For the induction of anesthesia prior to the use of other general anesthetic agents and for induction of anesthesia for short surgical, diagnostic, or therapeutic procedures associated with minimal painful stimuli. |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | They cause slurred speech, disorientation and "drunken" behavior. They are physically and psychologically addictive. They cause slurred speech, disorientation and "drunken" behavior. They are physically and psychologically addictive. |
|---|
| Symptoms | Symptoms of an overdose typically include sluggishness, incoordination, difficulty in thinking, slowness of speech, faulty judgment, drowsiness or coma, shallow breathing, staggering, and in severe cases coma and death. |
|---|
| Treatment | Not Available |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB01355 |
|---|
| HMDB ID | HMDB15444 |
|---|
| PubChem Compound ID | 3608 |
|---|
| ChEMBL ID | CHEMBL7728 |
|---|
| ChemSpider ID | 3482 |
|---|
| KEGG ID | C11723 |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | 5706 |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Hexobarbital |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Hexobarbital |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | T3D3039.pdf |
|---|
| General References | - Takenoshita R, Toki S: [New aspects of hexobarbital metabolism: stereoselective metabolism, new metabolic pathway via GSH conjugation, and 3-hydroxyhexobarbital dehydrogenases]. Yakugaku Zasshi. 2004 Dec;124(12):857-71. [15577260 ]
- Wahlstrom G: A study of the duration of acute tolerance induced with hexobarbital in male rats. Pharmacol Biochem Behav. 1998 Apr;59(4):945-8. [9586853 ]
- Korkmaz S, Ljungblad E, Wahlstrom G: Interaction between flumazenil and the anesthetic effects of hexobarbital in the rat. Brain Res. 1995 Apr 10;676(2):371-7. [7614008 ]
- Dall V, Orntoft U, Schmidt A, Nordholm L: Interaction of the competitive AMPA receptor antagonist NBQX with hexobarbital. Pharmacol Biochem Behav. 1993 Sep;46(1):73-6. [8255925 ]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|