| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2009-07-21 20:28:58 UTC |
|---|
| Update Date | 2014-12-24 20:25:56 UTC |
|---|
| Accession Number | T3D3064 |
|---|
| Identification |
|---|
| Common Name | Chlophedianol |
|---|
| Class | Small Molecule |
|---|
| Description | Chlophedianol is only found in individuals that have used or taken this drug. It is a centrally-acting cough suppressant available in Canada under the trade name Ulone. It is not available in the United States. Chlophedianol suppresses the cough reflex by a direct effect on the cough center in the medulla of the brain. |
|---|
| Compound Type | - Amine
- Anesthetic, Local
- Antitussive Agent
- Drug
- Histamine Antagonist
- Metabolite
- Organic Compound
- Organochloride
- Synthetic Compound
|
|---|
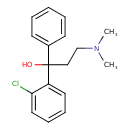
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | 1-(2-Chlorophenyl)-3-(dimethylamino)-1-phenyl-1-propanol | | 1-Phenyl-1-(O-chlorophenyl)-3-dimethylaminopropanol | | 2-Chloro-alpha-(2-(dimethylamino)ethyl)benzhydrol | | alpha-(Dimethylaminoethyl)-O-chlorobenzhydrol | | Antitussin | | Chlofedanol | | Clofedano | | Clofedanol | | Clofedanolum | | Clofedianolo | | Clophedianol base | | Ulone |
|
|---|
| Chemical Formula | C17H20ClNO |
|---|
| Average Molecular Mass | 289.800 g/mol |
|---|
| Monoisotopic Mass | 289.123 g/mol |
|---|
| CAS Registry Number | 791-35-5 |
|---|
| IUPAC Name | 1-(2-chlorophenyl)-3-(dimethylamino)-1-phenylpropan-1-ol |
|---|
| Traditional Name | baltix |
|---|
| SMILES | CN(C)CCC(O)(C1=CC=CC=C1)C1=CC=CC=C1Cl |
|---|
| InChI Identifier | InChI=1/C17H20ClNO/c1-19(2)13-12-17(20,14-8-4-3-5-9-14)15-10-6-7-11-16(15)18/h3-11,20H,12-13H2,1-2H3 |
|---|
| InChI Key | InChIKey=WRCHFMBCVFFYEQ-UHFFFAOYNA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as diphenylmethanes. Diphenylmethanes are compounds containing a diphenylmethane moiety, which consists of a methane wherein two hydrogen atoms are replaced by two phenyl groups. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Diphenylmethanes |
|---|
| Direct Parent | Diphenylmethanes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Diphenylmethane
- Chlorobenzene
- Aralkylamine
- Halobenzene
- Aryl chloride
- Aryl halide
- 1,3-aminoalcohol
- Tertiary alcohol
- Tertiary aliphatic amine
- Tertiary amine
- Organic nitrogen compound
- Organonitrogen compound
- Organochloride
- Organohalogen compound
- Organooxygen compound
- Aromatic alcohol
- Hydrocarbon derivative
- Organopnictogen compound
- Organic oxygen compound
- Amine
- Alcohol
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | 120°C | | Boiling Point | Not Available | | Solubility | 6.21e-02 g/L | | LogP | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0a4i-6920000000-9663bc4c9b4c6cddf803 | 2017-09-01 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-000l-3190000000-c824c404a084a83de8cd | 2017-10-06 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-002f-0490000000-3b2ca74cacc7304a7ac4 | 2016-08-02 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0007-0290000000-6bbcb1c25f0ce2866aa3 | 2016-08-02 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a7i-3900000000-14ead6824a79b1c917d0 | 2016-08-02 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0090000000-f2501d0b07fcc1bbf258 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-002r-6190000000-130788ec7458ac9f9483 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00or-9450000000-03b65fc4b4d7b5b0a9df | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0090000000-14dbfe1b830a461833ee | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-052f-2090000000-f53ebc7ce557c3f61718 | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a6r-9340000000-65e2916096fe866007e2 | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0090000000-3833a1b9bfc0387cd5b7 | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-3390000000-563ec7bd3091dfff650a | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001i-9570000000-b5963bbd2197b868f196 | 2021-10-11 | View Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-0pb9-9560000000-539851d74afb04d60422 | 2014-09-20 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Oral |
|---|
| Mechanism of Toxicity | Suppresses the cough reflex by a direct effect on the cough center in the medulla of the brain. |
|---|
| Metabolism | Hepatic. |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | Used in the treatment of dry cough. |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | May have anticholinergic effects at high doses. |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB04837 |
|---|
| HMDB ID | HMDB15585 |
|---|
| PubChem Compound ID | 2795 |
|---|
| ChEMBL ID | Not Available |
|---|
| ChemSpider ID | 2693 |
|---|
| KEGG ID | Not Available |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | 775264 |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Chlophedianol |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Clofedanol |
|---|
| References |
|---|
| Synthesis Reference | Lorenz, R., Gosswald, R. and Henecka, H.; US. Patent 3,031,377; April 24, 1962; assigned
to Farbenfabriken Bayer AG, Germany. |
|---|
| MSDS | Not Available |
|---|
| General References | - Wishart DS, Knox C, Guo AC, Cheng D, Shrivastava S, Tzur D, Gautam B, Hassanali M: DrugBank: a knowledgebase for drugs, drug actions and drug targets. Nucleic Acids Res. 2008 Jan;36(Database issue):D901-6. Epub 2007 Nov 29. [18048412 ]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|