| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2009-07-21 20:28:59 UTC |
|---|
| Update Date | 2014-12-24 20:25:56 UTC |
|---|
| Accession Number | T3D3066 |
|---|
| Identification |
|---|
| Common Name | Bepotastine |
|---|
| Class | Small Molecule |
|---|
| Description | Bepotastine is a non-sedating, selective antagonist of the histamine 1 (H1) receptor. Bepotastine was approved in Japan for use in the treatment of allergic rhinitis and uriticaria/puritus in July 2000 and January 2002, respectively, and is marketed by Tanabe Seiyaku Co., Ltd. under the brand name Talion. It is available in oral and opthalmic dosage forms in Japan. The opthalmic solution is FDA approved since Sept 8, 2009 and is under the brand name Bepreve. |
|---|
| Compound Type | - Amine
- Anti-Allergic Agent
- Drug
- Ether
- Histamine H1 Antagonist, Non-Sedating
- Mast Cell Stabilizer
- Metabolite
- Organic Compound
- Organochloride
- Synthetic Compound
|
|---|
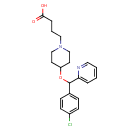
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | Bepreve | | Talion | | TAU-284DS |
|
|---|
| Chemical Formula | C21H25ClN2O3 |
|---|
| Average Molecular Mass | 388.888 g/mol |
|---|
| Monoisotopic Mass | 388.155 g/mol |
|---|
| CAS Registry Number | 125602-71-3 |
|---|
| IUPAC Name | 4-{4-[(4-chlorophenyl)(pyridin-2-yl)methoxy]piperidin-1-yl}butanoic acid |
|---|
| Traditional Name | talion |
|---|
| SMILES | OC(=O)CCCN1CCC(CC1)OC(C1=CC=C(Cl)C=C1)C1=CC=CC=N1 |
|---|
| InChI Identifier | InChI=1/C21H25ClN2O3/c22-17-8-6-16(7-9-17)21(19-4-1-2-12-23-19)27-18-10-14-24(15-11-18)13-3-5-20(25)26/h1-2,4,6-9,12,18,21H,3,5,10-11,13-15H2,(H,25,26) |
|---|
| InChI Key | InChIKey=YWGDOWXRIALTES-UHFFFAOYNA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as benzylethers. These are aromatic ethers with the general formula ROCR' (R = alkyl, aryl; R'=benzene). |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Benzylethers |
|---|
| Direct Parent | Benzylethers |
|---|
| Alternative Parents | |
|---|
| Substituents | - Benzylether
- Amino fatty acid
- Chlorobenzene
- Halobenzene
- Aryl chloride
- Aryl halide
- Fatty acyl
- Pyridine
- Piperidine
- Heteroaromatic compound
- Amino acid or derivatives
- Amino acid
- Tertiary aliphatic amine
- Tertiary amine
- Carboxylic acid derivative
- Carboxylic acid
- Azacycle
- Dialkyl ether
- Ether
- Organoheterocyclic compound
- Monocarboxylic acid or derivatives
- Organic oxygen compound
- Organic nitrogen compound
- Amine
- Carbonyl group
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Organohalogen compound
- Organochloride
- Organonitrogen compound
- Organooxygen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | - Cytoplasm
- Extracellular
- Membrane
|
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | |
|---|
| Biological Roles | |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | 5.03e-02 g/L | | LogP | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0udi-2292000000-5b45fd7805bbbd416e7f | 2017-09-01 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-0udi-2192000000-0ea685d018da4a2938de | 2017-10-06 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0079-0109000000-324c9088d0c2cb4aac38 | 2016-06-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00du-1319000000-6f5aa9888fbcca679b0d | 2016-06-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00du-9820000000-c0621adfa83f3477b8ab | 2016-06-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0019000000-90b8af4e7923949436c2 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00kr-0129000000-4cc6ef36f76266a06698 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014i-3690000000-f62361da241614f257f0 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0009000000-dfc0e49e18673a296c13 | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0uki-0049000000-192d682169678552ae49 | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0uk9-4940000000-4571714c90c45dfd6f35 | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0009000000-2f4b05915f2ae5688d7f | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00ku-2119000000-3967902f22f168d22051 | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014i-5490000000-80c10183c44faf9ff027 | 2021-10-11 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Oral, Ophthalmic. Tmax, after single dose, opthalmic = 1.2 hours;
Cmax, 1.5%, opthalmic dose = 7.3 ± 1.9 ng/mL;
After 24 hours post-installation, levels of bepotastine are below quantifiable limit of 2 ng/mL. Minimal systemic absorption with opthalmic dosage form. |
|---|
| Mechanism of Toxicity | Because of a type 1 hypersensitivity reaction cascade that is triggered by antigen exposure, allergic conjunctivitis occurs. Allergen exposure is followed by conjunctival mast cell degranulation and histamine released as a result of the formation of complementary IgE cross-links on the conjunctiva. Due to the release of histamine, symptoms such as itching can be observed. Bepotastine works to relieve itchy eyes by three primary mechanisms of action. It is a non-sedating, selective antagonist of the histamine 1 (H1) receptor, a mast cell stabilizer, and it suppresses the migration of eosinophils into inflamed tissues to prevent tissue damage and worsening of allergic inflammation of the conjunctiva. |
|---|
| Metabolism | Minimal metabolism via CYP enzymes
Route of Elimination: When a oral dose of 2.5 - 40 mg bepotastine is given, 75%-90% of the dose was excreted unchanged in the urine by 24 hours.
Half Life: Elimination half life = 2.5 hours |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | For the symptomatic treatment of itchy eyes (caused by IgE-induced mast cell degranulation) due to allergic conjunctivitis. Used in the treatment of allergic rhinitis and uriticaria/puritus. [Wikipedia] |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB04890 |
|---|
| HMDB ID | HMDB15600 |
|---|
| PubChem Compound ID | 2350 |
|---|
| ChEMBL ID | CHEMBL1201758 |
|---|
| ChemSpider ID | 2260 |
|---|
| KEGG ID | Not Available |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | 71204 |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Bepotastine |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Bepotastine |
|---|
| References |
|---|
| Synthesis Reference | Tae Hee Ha, Chang Hee Park, Won Jeoung Kim, Soohwa Cho, Han Kyong Kim, Kwee Hyun Suh, “PROCESS FOR PREPARING BEPOTASTINE AND INTERMEDIATES USED THEREIN.” U.S. Patent US20100168433, issued July 01, 2010. |
|---|
| MSDS | T3D3066.pdf |
|---|
| General References | - Ohashi R, Kamikozawa Y, Sugiura M, Fukuda H, Yabuuchi H, Tamai I: Effect of P-glycoprotein on intestinal absorption and brain penetration of antiallergic agent bepotastine besilate. Drug Metab Dispos. 2006 May;34(5):793-9. Epub 2006 Feb 2. [16455807 ]
- Andoh T, Kuraishi Y: Suppression by bepotastine besilate of substance P-induced itch-associated responses through the inhibition of the leukotriene B4 action in mice. Eur J Pharmacol. 2006 Oct 10;547(1-3):59-64. Epub 2006 Jul 25. [16914135 ]
- Simons FE, Simons KJ: Histamine and H1-antihistamines: celebrating a century of progress. J Allergy Clin Immunol. 2011 Dec;128(6):1139-1150.e4. doi: 10.1016/j.jaci.2011.09.005. Epub 2011 Oct 27. [22035879 ]
- Wingard JB, Mah FS: Critical appraisal of bepotastine in the treatment of ocular itching associated with allergic conjunctivitis. Clin Ophthalmol. 2011;5:201-7. doi: 10.2147/OPTH.S8665. Epub 2011 Feb 15. [21386912 ]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|