| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2009-07-30 17:58:38 UTC |
|---|
| Update Date | 2014-12-24 20:26:07 UTC |
|---|
| Accession Number | T3D3480 |
|---|
| Identification |
|---|
| Common Name | Atenolol |
|---|
| Class | Small Molecule |
|---|
| Description | Atenolol is a so-called beta1-selective (or 'cardioselective') drug. That means that it exerts greater blocking activity on myocardial beta1-receptors than on beta2 ones in the lung. The beta2 receptors are responsible to keep the bronchial system open. If these receptors are blocked, bronchospasm with serious lack of oxygen in the body can result. However, due to its cardioselective properties, the risk of bronchospastic reactions if using atenolol is reduced compared to nonselective drugs as propranolol. Nonetheless, this reaction may also be encountered with atenolol, particularly with high doses. Extreme caution should be exerted if atenolol is given to asthma patients, who are particularly at risk; the dose should be as low as possible. If an asthma attack occurs, the inhalation of a beta2-mimetic antiasthmatic, such as hexoprenalin or salbutamol, will usually suppress the symptoms. Atenolol (trade name Tenormin) can be used to treat cardiovascular diseases such as hypertension, coronary heart disease, arrhythmias, and treatment of myocardial infarction after the acute event. Patients with compensated congestive heart failure may be treated with atenolol as a co medication (usually together with an ACE inhibitor, a diuretic and a digitalis-glycoside, if indicated). In patients with congestive heart failure, it reduces the need for and the consumption of oxygen of the heart muscle. It is very important to start with low doses, as atenolol reduces also the muscular power of the heart, which is an undesired effect in congestive heart failure. |
|---|
| Compound Type | - Adrenergic Agent
- Adrenergic beta-1 Receptor Antagonist
- Adrenergic beta-Antagonist
- Amide
- Amine
- Anti-Arrhythmia Agent
- Antihypertensive Agent
- Drug
- Ether
- Food Toxin
- Metabolite
- Organic Compound
- Sympatholytic
- Synthetic Compound
|
|---|
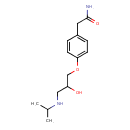
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | 1-p-Carbamoylmethylphenoxy-3-isopropylamino-2-propanol | | 2-(p-(2-Hydroxy-3-(isopropylamino)propoxy)phenyl)acetamide | | 4-(2-Hydroxy-3-((1-methylethyl)amino)propoxy)benzeneacetamide | | Aircrit | | Alinor | | Altol | | Anselol | | Antipressan | | Apo-Atenolol | | Atcardil | | Atecard | | Atehexal | | Atenblock | | Atendol | | Atenet | | Ateni | | Atenil | | Atenol 1A pharma | | Atenol acis | | Atenol AL | | Atenol Atid | | Atenol Cophar | | Atenol ct | | Atenol Fecofar | | Atenol Gador | | Atenol Genericon | | Atenol GNR | | Atenol Heumann | | Atenol MSD | | Atenol NM Pharma | | Atenol Nordic | | Atenol PB | | Atenol Quesada | | Atenol Stada | | Atenol Tika | | Atenol Trom | | Atenol von ct | | Atenol-Mepha | | Atenol-ratiopharm | | Atenol-Wolff | | Atenolin | | Atenololum | | Atenomel | | Atereal | | Aterol | | Betablok | | Betacard | | Betasyn | | Betatop Ge | | Blocotenol | | Blokium | | Cardaxen | | Cardiopress | | Corotenol | | Cuxanorm | | Duraatenolol | | Duratenol | | Evitocor | | Farnormin | | Felo-Bits | | Hipres | | Hypoten | | Ibinolo | | Internolol | | Jenatenol | | Juvental | | Lo-ten | | Loten | | Lotenal | | Myocord | | Normalol | | Normiten | | Noten | | Oraday | | Ormidol | | Panapres | | Plenacor | | Premorine | | Prenolol | | Prenormine | | Prinorm | | Scheinpharm Atenol | | Seles beta | | Selobloc | | Serten | | Servitenol | | Stermin | | Tenidon | | Tenobloc | | Tenoblock | | Tenolol | | Tenoprin | | Tenoretic | | Tenormin | | Tenormine | | Tensimin | | Tredol | | Unibloc | | Uniloc | | Vascoten | | Vericordin | | Wesipin | | Xaten |
|
|---|
| Chemical Formula | C14H22N2O3 |
|---|
| Average Molecular Mass | 266.336 g/mol |
|---|
| Monoisotopic Mass | 266.163 g/mol |
|---|
| CAS Registry Number | 29122-68-7 |
|---|
| IUPAC Name | 2-(4-{2-hydroxy-3-[(propan-2-yl)amino]propoxy}phenyl)acetamide |
|---|
| Traditional Name | atenolol |
|---|
| SMILES | CC(C)NCC(O)COC1=CC=C(CC(O)=N)C=C1 |
|---|
| InChI Identifier | InChI=1/C14H22N2O3/c1-10(2)16-8-12(17)9-19-13-5-3-11(4-6-13)7-14(15)18/h3-6,10,12,16-17H,7-9H2,1-2H3,(H2,15,18) |
|---|
| InChI Key | InChIKey=METKIMKYRPQLGS-UHFFFAOYNA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as phenylacetamides. These are amide derivatives of phenylacetic acids. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Phenylacetamides |
|---|
| Direct Parent | Phenylacetamides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Phenylacetamide
- Phenoxy compound
- Phenol ether
- Alkyl aryl ether
- 1,2-aminoalcohol
- Amino acid or derivatives
- Carboxamide group
- Secondary alcohol
- Primary carboxylic acid amide
- Carboxylic acid derivative
- Secondary amine
- Secondary aliphatic amine
- Ether
- Organic nitrogen compound
- Organonitrogen compound
- Organooxygen compound
- Hydrocarbon derivative
- Organic oxide
- Carbonyl group
- Organopnictogen compound
- Amine
- Organic oxygen compound
- Alcohol
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | |
|---|
| Biological Roles | |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | 158-160°C | | Boiling Point | Not Available | | Solubility | 1.33E+004 mg/L (at 25°C) | | LogP | 0.16 |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0kmi-8920000000-75c70fad839dc47116bf | 2017-08-28 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-05fr-9451000000-8069c1e0c4bc41c41dc5 | 2017-10-06 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-0uy0-0950000000-e882b8032954e2a624cf | 2012-07-25 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-0076-4920000000-3c7f23b7695e5c4dcc4c | 2012-07-25 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-0aos-9800000000-93a18f0e030605aa10aa | 2012-07-25 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-qTof , Positive | splash10-0002-3910000000-43ae37638a8827ea76f1 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-01b9-6980000000-3a2af9caa0ddc645756a | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-014i-0290000000-0e1f60f7eeef06627206 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-0002-0900000000-dda902f75e8d8e525d4f | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-0002-0900000000-32b90aea04b68bdedbef | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-0002-0900000000-f333c3bfc9bd6c0593d4 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , positive | splash10-004l-0890000000-5c0a8df3cdde41f290f2 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , positive | splash10-014i-0090000000-58fd12e8cca5ee837799 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , positive | splash10-014i-0090000000-271732f56fff3c9ecf87 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , positive | splash10-014l-3960000000-ee2e8e341081d16f5fdb | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , positive | splash10-006t-3900000000-ff916216d2f4fc373c18 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , positive | splash10-007k-3900000000-7ce05e231c161678a1d9 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , positive | splash10-0a5a-4900000000-4d9ba2cbcfaa9a4fd7fc | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , positive | splash10-014i-0090000000-326ded7fdf97f0b34fc5 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , positive | splash10-014i-0090000000-bb7e0317c8b0c0a5abbc | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , positive | splash10-014l-3960000000-7c774d4cb55b5368392a | 2017-09-14 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0gb9-1190000000-9c46f6de428fd9c85b0c | 2017-07-25 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-5490000000-d3676c10983a2bf51eb7 | 2017-07-25 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05fr-9400000000-f879d668e69afad6eb16 | 2017-07-25 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-1590000000-21fbf983e89a88c8fda0 | 2017-07-26 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0zfr-2910000000-55258981df3fe8b9c9ed | 2017-07-26 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9500000000-fc86b722c900f0c12c6f | 2017-07-26 | View Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-00e9-9000000000-6a38697da7945fdb1908 | 2014-09-20 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 600 MHz, H2O, experimental) | Not Available | 2012-12-05 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 400 MHz, DMSO-d6, experimental) | Not Available | 2014-09-20 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 22.53 MHz, DMSO-d6, experimental) | Not Available | 2014-09-23 | View Spectrum | | 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, H2O, experimental) | Not Available | 2012-12-05 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Approximately 50% of an oral dose is absorbed from the gastrointestinal tract, the remainder being excreted unchanged in the feces. |
|---|
| Mechanism of Toxicity | Like metoprolol, atenolol competes with sympathomimetic neurotransmitters such as catecholamines for binding at beta(1)-adrenergic receptors in the heart and vascular smooth muscle, inhibiting sympathetic stimulation. This results in a reduction in resting heart rate, cardiac output, systolic and diastolic blood pressure, and reflex orthostatic hypotension. Higher doses of atenolol also competitively block beta(2)-adrenergic responses in the bronchial and vascular smooth muscles. |
|---|
| Metabolism | Hepatic (minimal)
Route of Elimination: Approximately 50% of an oral dose is absorbed from the gastrointestinal tract, the remainder being excreted unchanged in the feces. Unlike propranolol or metoprolol, but like nadolol, atenolol undergoes little or no metabolism by the liver, and the absorbed portion is eliminated primarily by renal excretion.
Half Life: 6-7 hours |
|---|
| Toxicity Values | LD50: 2000-3000 mg/kg(oral, mice). |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | For the management of hypertention and long-term management of patients with angina pectoris |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Symptoms of an atenolol overdose include a slow heart beat, shortness of breath, fainting, dizziness, weakness, confusion, nausea, and vomiting. |
|---|
| Treatment | Not Available |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB00335 |
|---|
| HMDB ID | HMDB01924 |
|---|
| PubChem Compound ID | 2249 |
|---|
| ChEMBL ID | CHEMBL24 |
|---|
| ChemSpider ID | 2162 |
|---|
| KEGG ID | C13235 |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | 2904 |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Atenolol |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Atenolol |
|---|
| References |
|---|
| Synthesis Reference | Barrett, A.M., Carter, J., Hull, R., Le Count, D.J. and Squire, C.J.; U.S. Patent 3,663,607;
May 16, 1972; assigned to Imperial Chemical Industries Limited, England.
Barrett, A.M., Carter, J., Hull, R., Le Count, D.J. and Squire, C.J.; U.S. Patent 3,836,671;
September 17, 1974; assigned to Imperial Chemical Industries Limited, England. |
|---|
| MSDS | Link |
|---|
| General References | - Drugs.com [Link]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | | Gene | Gene Symbol | Gene ID | Interaction | Chromosome | Details |
|---|
|
|---|
| Down-Regulated Genes | | Gene | Gene Symbol | Gene ID | Interaction | Chromosome | Details |
|---|
|
|---|