| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2009-07-30 17:59:01 UTC |
|---|
| Update Date | 2014-12-24 20:26:07 UTC |
|---|
| Accession Number | T3D3520 |
|---|
| Identification |
|---|
| Common Name | Melamine |
|---|
| Class | Small Molecule |
|---|
| Description | Melamine is an organic base and a trimer of cyanamide, with a 1,3,5-triazine skeleton. Like cyanamide, it contains 66% nitrogen by mass and, if mixed with resins, has fire retardant properties due to its release of nitrogen gas when burned or charred, and has several other industrial uses. Melamine is also a metabolite of cyromazine, a pesticide. It is formed in the body of mammals who have ingested cyromazine. It has been reported that cyromazine can also be converted to melamine in plants. Melamine is described as Harmful if swallowed, inhaled or absorbed through the skin. Chronic exposure may cause cancer or reproductive damage. Eye, skin and respiratory irritant. However, the short-term lethal dose is on a par with common table salt with an LD50 of more than 3 grams per kilogram of bodyweight.[15] U.S. Food and Drug Administration (FDA) scientists explained that when melamine and cyanuric acid are absorbed into the bloodstream, they concentrate and interact in the urine-filled renal tubules, then crystallize and form large numbers of round, yellow crystals, which in turn block and damage the renal cells that line the tubes, causing the kidneys to malfunction. |
|---|
| Compound Type | - Amine
- Aromatic Hydrocarbon
- Food Toxin
- Household Toxin
- Industrial Precursor/Intermediate
- Industrial/Workplace Toxin
- Lachrymator
- Metabolite
- Organic Compound
- Pesticide
- Synthetic Compound
|
|---|
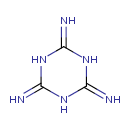
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | 1,3, 5-Triazine-2,4,6-triamine | | 1,3,5-Triazine-2,4,6(1H,3H,5H)-triimine | | 1,3,5-Triazine-2,4,6-triamine | | 2,4, 6-Triamino-1,3,5-triazine | | 2,4,6-Triamino-1,3,5-triazine | | 2,4,6-Triamino-S-Triazine | | 2,4,6-Triaminotriazine | | 2,4,6-Tris(1-aziridinyl)-S-triazine | | 4,6-Diamino-1,2-dihydro-2-imino-S-Triazine | | ADK Stab ZS 27 | | Aero | | Cyanuramide | | Cyanuric triamide | | Cyanurotriamide | | Cyanurotriamine | | Cyanurtriamide | | Cymel | | DG 002 (amine) | | Hicophor PR | | Isomelamine | | Mark ZS 27 | | Pluragard | | Pluragard C 133 | | S-Triazinetriamine | | Spinflam ML 94M | | Sym-triaminotriazine | | Teoharn | | Theoharn | | Triaminotriazine | | Virset 656-4 | | Yukamelamine |
|
|---|
| Chemical Formula | C3H6N6 |
|---|
| Average Molecular Mass | 126.120 g/mol |
|---|
| Monoisotopic Mass | 126.065 g/mol |
|---|
| CAS Registry Number | 108-78-1 |
|---|
| IUPAC Name | 1,3,5-triazinane-2,4,6-triimine |
|---|
| Traditional Name | melamine |
|---|
| SMILES | N=C1NC(=N)NC(=N)N1 |
|---|
| InChI Identifier | InChI=1S/C3H6N6/c4-1-7-2(5)9-3(6)8-1/h(H6,4,5,6,7,8,9) |
|---|
| InChI Key | InChIKey=JDSHMPZPIAZGSV-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 1,3,5-triazines. 1,3,5-triazines are compounds containing a triazine ring, which is a heterocyclic ring, similar to the six-member benzene ring but with three carbons replaced by nitrogen atoms, at ring positions 1, 3, and 5. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Triazines |
|---|
| Sub Class | 1,3,5-triazines |
|---|
| Direct Parent | 1,3,5-triazines |
|---|
| Alternative Parents | |
|---|
| Substituents | - 1,3,5-triazine
- Heteroaromatic compound
- Azacycle
- Organic nitrogen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Organonitrogen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. (1) |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | 345°C | | Boiling Point | > 280 °C | | Solubility | 3.24 mg/mL at 20°C | | LogP | -1.37 |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-004i-3900000000-71dbc7269c93dda90b2c | 2017-09-01 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-004i-0900000000-a41f1e20c8ec4934d360 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-004i-0900000000-ae6d1e3320b13071b833 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , positive | splash10-004i-0900000000-206bb0a304c5a8440070 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , positive | splash10-004i-1900000000-7e8a77b9e84e57bd7cef | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , positive | splash10-004i-1900000000-8e0523cbbcafd9f9db92 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , positive | splash10-004i-4900000000-e45e493846516172500b | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , positive | splash10-002r-9600000000-a6d255dfed4fa6c2633f | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , positive | splash10-000i-9200000000-d8433e44aa15ddcbc086 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-004i-1900000000-738eb18fe28cac903509 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-000i-9200000000-dae6ee5dfe2d8ac93f80 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-014l-9000000000-fc1d6d3837aeaab95233 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , positive | splash10-004i-1900000000-91f26f642ce9612598a1 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 90V, Positive | splash10-000i-9200000000-d8433e44aa15ddcbc086 | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 75V, Positive | splash10-002r-9600000000-a6d255dfed4fa6c2633f | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 35V, Positive | splash10-004i-1900000000-838ef9febd7e2e0b2fea | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 45V, Positive | splash10-004i-1900000000-736ea73ed36f89306de1 | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 30V, Positive | splash10-004i-0900000000-2c40f1fca432608a00d3 | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 30V, Positive | splash10-004i-0900000000-00f72d1e1bb88e391cb8 | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 40V, Positive | splash10-014l-9000000000-ab81cf47f2116f7fa324 | 2021-09-20 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0900000000-3e8b6a1a6fdb2209d0ea | 2016-08-02 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004i-0900000000-d113db88468963a77c18 | 2016-08-02 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9200000000-e1ef56b1bf95f453711f | 2016-08-02 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0900000000-1bfb2f0efa2647a0552d | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-002f-9600000000-9cf3208803e0b283879f | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000x-9000000000-425ba1390c5b2c68ebe3 | 2016-08-03 | View Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-004i-9800000000-c6f386c1797fc9995734 | 2014-09-20 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 15.08 MHz, DMSO-d6, experimental) | Not Available | 2014-09-23 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Oral (1) ; inhalation (1) ; dermal (1) |
|---|
| Mechanism of Toxicity | Melamine causes carcinomas of the urinary bladder at high doses (in male rats). Formation of bladder stones occurred and these calculi are necessary for the induction of tumours. Carcinomas are induced by continuous irritation of the bladder epithelium by the calculi, so that melamine acts only indirectly as a non-genotoxic carcinogen. (2) |
|---|
| Metabolism | Melamine is not metabolized and is rapidly eliminated via urine in a study with oral application to rats. (2) |
|---|
| Toxicity Values | LD50: 3161 mg/kg (Oral, Rat) (2)
LD50: 3296 mg/kg (Oral, Mouse) (2)
LD50: > 1000 mg/kg (Dermal, Rabbit) (2)
LC50: 3248 mg/m3 (Inhalation, Rat) (2) |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | 3, not classifiable as to its carcinogenicity to humans. (4) |
|---|
| Uses/Sources | Melamine has many industrial uses, including the production of laminates, glues, adhesives, moulding compounds, coatings and flame retardants. It is also used in some fertilizers and in the production of melamine resins, typically by reaction with formaldehyde. Melamine is a metabolite of the pesticide cyromazine in plants, goats, hens and rats. (1) (3) |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Studies ranging from skin irritation to carcinogenicity are available. Melamine is not genotoxic but it causes carcinomas of the urinary bladder at high doses. Melamine is not irritating to skin and eye, not sensitising and not teratogenic. (2) |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB41922 |
|---|
| PubChem Compound ID | 7955 |
|---|
| ChEMBL ID | CHEMBL1231106 |
|---|
| ChemSpider ID | 7667 |
|---|
| KEGG ID | C08737 |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | 27915 |
|---|
| BioCyc ID | DIHYDRO-DIOH-BENZOATE |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Melamine |
|---|
| PDB ID | AX2 |
|---|
| ACToR ID | 817 |
|---|
| Wikipedia Link | Melamine |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Link |
|---|
| General References | - Wikipedia. Melamine. Last updated 10 August 2009. [Link]
- International Programme on Chemical Safety (IPCS) INCHEM (2002). OECD Screening Information DataSet (SIDS). High Production Volume Chemicals. Melamine. [Link]
- World Health Organization (2008). Melamine and Cyanuric acid: toxicity, preliminary risk assessment and guidance on levels in food. [Link]
- International Agency for Research on Cancer (2014). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. [Link]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | | Gene | Gene Symbol | Gene ID | Interaction | Chromosome | Details |
|---|
|
|---|
| Down-Regulated Genes | Not Available |
|---|