| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2010-04-16 22:10:27 UTC |
|---|
| Update Date | 2014-12-24 20:26:21 UTC |
|---|
| Accession Number | T3D3681 |
|---|
| Identification |
|---|
| Common Name | Cytochalasin J |
|---|
| Class | Small Molecule |
|---|
| Description | Cytochalasins are mycotoxins that have the ability to bind to actin filaments and block polymerization and the elongation of actin. As a result, they can change cellular morphology, inhibit cellular processes such as cell division, and cause cells to undergo apoptosis. Cytochalasins also have the ability to permeate cell membranes, prevent cellular translocation, cause cells to enucleate, and affect other aspects of biological processes unrelated to actin polymerization. Cytochalasin J is has been isolated from the fungus Phomopsis paspali. It also has shown CNS activity. (1, 2, 6, 7) |
|---|
| Compound Type | - Amide
- Amine
- Fungal Toxin
- Mycotoxin
- Natural Compound
- Organic Compound
|
|---|
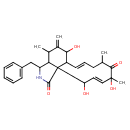
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | (3S,3aR,4S,6aR,7E,13E,15R)-3-Benzyl-6,12,15-trihydroxy-4,10,12-trimethyl-5-methylene-2,3,3a,4,5,6,6a,9,10,11,12,15-dodecahydro-1H-cycloundeca[d]isoindol-1-one | | 7,18,21-Trihydroxy-16,18-dimethyl-10-phenyl-(11)cytochalasa-6(12),13,19-trien-1-one | | Kodocytochalasin 2 | | Paspalin P | | Paspalin P II |
|
|---|
| Chemical Formula | C28H35NO5 |
|---|
| Average Molecular Mass | 465.581 g/mol |
|---|
| Monoisotopic Mass | 465.252 g/mol |
|---|
| CAS Registry Number | 56144-22-0 |
|---|
| IUPAC Name | 3-benzyl-6,12,15-trihydroxy-4,10,12-trimethyl-5-methylidene-1H,2H,3H,4H,5H,6H,6aH,9H,10H,11H,12H,15H,15bH-cycloundeca[e]isoindole-1,11-dione |
|---|
| Traditional Name | 3-benzyl-6,12,15-trihydroxy-4,10,12-trimethyl-5-methylidene-2H,3H,4H,6H,6aH,9H,10H,15H,15bH-cycloundeca[e]isoindole-1,11-dione |
|---|
| SMILES | CC1C2C(CC3=CC=CC=C3)NC(=O)C22C(\C=C\CC(C)C(=O)C(C)(O)\C=C\C2O)C(O)C1=C |
|---|
| InChI Identifier | InChI=1S/C28H35NO5/c1-16-9-8-12-20-24(31)18(3)17(2)23-21(15-19-10-6-5-7-11-19)29-26(33)28(20,23)22(30)13-14-27(4,34)25(16)32/h5-8,10-14,16-17,20-24,30-31,34H,3,9,15H2,1-2,4H3,(H,29,33)/b12-8+,14-13+ |
|---|
| InChI Key | InChIKey=DMUBZPWTFAPROZ-KRQHZRJMSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as cytochalasans. These are fungal metabolites structurally characterized by the presence of an isoindolone nucleus fused to a macrocyclic ring, which can either a lactone, as in cytochalasin B, a carbonate, as in cytochalasin E, or a carbocycle, as in cytochalasin D, H, and K. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Alkaloids and derivatives |
|---|
| Class | Cytochalasans |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Cytochalasans |
|---|
| Alternative Parents | |
|---|
| Substituents | - Carbocyclic cytochalasan skeleton
- Cytochalasan
- Isoindolone
- Isoindoline
- Isoindole
- Isoindole or derivatives
- Acyloin
- Monocyclic benzene moiety
- Benzenoid
- 2-pyrrolidone
- Pyrrolidone
- Cyclic alcohol
- Tertiary alcohol
- Pyrrolidine
- Carboxamide group
- Cyclic ketone
- Secondary carboxylic acid amide
- Ketone
- Lactam
- Secondary alcohol
- Organoheterocyclic compound
- Azacycle
- Carboxylic acid derivative
- Polyol
- Alcohol
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Carbonyl group
- Organonitrogen compound
- Organooxygen compound
- Organic nitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | 137-139°C | | Boiling Point | Not Available | | Solubility | Not Available | | LogP | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0000900000-6166edd911e8c979c438 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001j-2004900000-62cb9f9ca17e29c2720a | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0zfu-9803000000-270a1dc60e3899a64971 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0000900000-93af0e3cf07ec39caa4f | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01ot-0001900000-6a135cdbebc3b471eb93 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9024200000-2d614b46a29c3eda8c8b | 2016-08-03 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Oral, dermal, inhalation, and parenteral (contaminated drugs). (3) |
|---|
| Mechanism of Toxicity | Cytochalasins are known to bind to the barbed, fast growing plus ends of microfilaments, which then blocks both the assembly and disassembly of individual actin monomers from the bound end. Once bound, cytochalasin essentially caps the end of the new actin filament. One cytochalasin will bind to one actin filament. By blocking the polymerization and elongation of actin, cytochalasins can change cellular morphology, inhibit cellular processes such as cell division, and cause cells to undergo apoptosis. (1, 2, 6) |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | Cytochalasin J is has been isolated from the fungus Phomopsis paspali. (7) |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Major biological effects of cytochalasins include inhibition of the division of cytoplasm, reversible inhibition of cell movement, induction of nuclear extrusion, inhibition of such processes as phagocytosis, platelet aggregation and clot retraction, glucose transport, thyroid secretion, and release of growth hormone. Some cytochalasins have been shown to have developmental effects. (7) |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Consider activated charcoal after gastrointestinal absportion. Nitroprusside is recommended to reverse peripheral ischemia secondary to vasoconstriction and for the treatment of hypertension. Anticoagulant therapy with intravenous heparin is also recommended. (4) |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| PubChem Compound ID | Not Available |
|---|
| ChEMBL ID | Not Available |
|---|
| ChemSpider ID | Not Available |
|---|
| KEGG ID | Not Available |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | - Haidle AM, Myers AG: An enantioselective, modular, and general route to the cytochalasins: synthesis of L-696,474 and cytochalasin B. Proc Natl Acad Sci U S A. 2004 Aug 17;101(33):12048-53. Epub 2004 Jun 18. [15208404 ]
- Cooper JA: Effects of cytochalasin and phalloidin on actin. J Cell Biol. 1987 Oct;105(4):1473-8. [3312229 ]
- Peraica M, Domijan AM: Contamination of food with mycotoxins and human health. Arh Hig Rada Toksikol. 2001 Mar;52(1):23-35. [11370295 ]
- Grond S, Sablotzki A: Clinical pharmacology of tramadol. Clin Pharmacokinet. 2004;43(13):879-923. [15509185 ]
- Rumack BH POISINDEX(R) Information System Micromedex, Inc., Englewood, CO, 2010; CCIS Volume 143, edition expires Feb, 2010. Hall AH & Rumack BH (Eds): TOMES(R) Information System Micromedex, Inc., Englewood, CO, 2010; CCIS Volume 143, edition expires Feb, 2010.
- Cytochalasin. Wikipedia. Last Updated 12 April 2010. [Link]
- Sigma Aldrich 1996. Technical Bulletin AL-126: The Cytochalasins. [Link]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|