| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2014-08-29 06:13:56 UTC |
|---|
| Update Date | 2014-12-24 20:26:45 UTC |
|---|
| Accession Number | T3D4282 |
|---|
| Identification |
|---|
| Common Name | 17-Hydroxyprogesterone |
|---|
| Class | Small Molecule |
|---|
| Description | It serves as an intermediate in the biosynthesis of hydrocortisone and gonadal steroid hormones. It is derived from progesterone via 17-hydroxylase, a P450c17 enzyme, or from 17-hydroxypregnenolone via 3β-hydroxysteroid dehydrogenase/Δ5-4 isomerase. 17-Hydroxyprogesterone is a natural progestin and in pregnancy increases in the third trimester primarily due to fetal adrenal production. This hormone is primarily produced in the adrenal glands and to some degree in the gonads, specifically the corpus luteum of the ovary. Normal levels are 3-90 ng/dl in children, and in women, 15-70 ng/dl prior to ovulation, and 35-290 ng/dl during the luteal phase. Measurements of levels of 17-hydroxyprogesterone are useful in the evaluation of patients with suspected congenital adrenal hyperplasia as the typical enzymes that are defective, namely 21-hydroxylase and 11β-hydroxylase, lead to a build-up of 17OHP. In contrast, the rare patient with 17α-hydroxylase deficiency will have very low or undetectable levels of 17OHP. 17OHP levels can also be used to measure contribution of progestational activity of the corpus luteum during pregnancy as progesterone but not 17OHP is also contributed by the placenta. |
|---|
| Compound Type | - Animal Toxin
- Ester
- Food Toxin
- Industrial/Workplace Toxin
- Metabolite
- Natural Compound
- Organic Compound
|
|---|
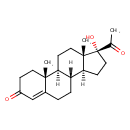
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | 17-alpha-Hydroxyprogesterone | | 17-Hydroxypregn-4-ene-3,20-dione | | 17-OH Progesterone | | 17-OHP | | 17a-Hydroxypregn-4-ene-3,20-dione | | 17a-Hydroxyprogesterone | | 17alpha-hydroxyprogesterone | | D4-Pregnen-17a-ol-3,20-dione | | Gestageno | | Gestageno Gador | | Hydroxyprogesterone | | Pregn-4-en-17a-ol-3,20-dione | | Prodix | | Prodox |
|
|---|
| Chemical Formula | C21H30O3 |
|---|
| Average Molecular Mass | 330.461 g/mol |
|---|
| Monoisotopic Mass | 330.219 g/mol |
|---|
| CAS Registry Number | 68-96-2 |
|---|
| IUPAC Name | (1S,2R,10R,11S,14R,15S)-14-acetyl-14-hydroxy-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadec-6-en-5-one |
|---|
| Traditional Name | (1S,2R,10R,11S,14R,15S)-14-acetyl-14-hydroxy-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadec-6-en-5-one |
|---|
| SMILES | [H][C@@]12CC[C@](O)(C(C)=O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CCC2=CC(=O)CC[C@]12C |
|---|
| InChI Identifier | InChI=1S/C21H30O3/c1-13(22)21(24)11-8-18-16-5-4-14-12-15(23)6-9-19(14,2)17(16)7-10-20(18,21)3/h12,16-18,24H,4-11H2,1-3H3/t16-,17+,18+,19+,20+,21+/m1/s1 |
|---|
| InChI Key | InChIKey=DBPWSSGDRRHUNT-CEGNMAFCSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as gluco/mineralocorticoids, progestogins and derivatives. These are steroids with a structure based on a hydroxylated prostane moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Pregnane steroids |
|---|
| Direct Parent | Gluco/mineralocorticoids, progestogins and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Progestogin-skeleton
- 20-oxosteroid
- 3-oxo-delta-4-steroid
- 3-oxosteroid
- 17-hydroxysteroid
- Oxosteroid
- Hydroxysteroid
- Delta-4-steroid
- Cyclohexenone
- Alpha-hydroxy ketone
- Cyclic alcohol
- Tertiary alcohol
- Cyclic ketone
- Ketone
- Organic oxygen compound
- Carbonyl group
- Hydrocarbon derivative
- Alcohol
- Organooxygen compound
- Organic oxide
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | - 17alpha-hydroxy steroid (CHEBI:17252 )
- Progestagens (C01176 )
- C21 steroids (gluco/mineralocorticoids, progestogins) and derivatives (LMST02030161 )
|
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Endogenous |
|---|
| Cellular Locations | - Cytoplasm
- Endoplasmic reticulum
- Extracellular
- Membrane
|
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | - Adipose Tissue
- Adrenal Cortex
- Adrenal Gland
- Testes
|
|---|
| Pathways | | Name | SMPDB Link | KEGG Link |
|---|
| Androgen and Estrogen Metabolism | SMP00068 | map00150 | | Steroidogenesis | SMP00130 | map00140 | | Adrenal Hyperplasia Type 3 or Congenital Adrenal Hyperplasia due to 21-hydroxylase Deficiency | SMP00373 | Not Available |
|
|---|
| Applications | Not Available |
|---|
| Biological Roles | |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | 219 - 220°C | | Boiling Point | Not Available | | Solubility | 0.00648 mg/mL | | LogP | 3.17 |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (2 MEOX; 1 TMS) | splash10-004l-4911000000-d40ba45835d1ee1b6c41 | 2014-06-16 | View Spectrum | | GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-002o-9651000000-b44110f9abcffa42ce35 | 2017-09-12 | View Spectrum | | GC-MS | GC-MS Spectrum - GC-MS (Non-derivatized) | splash10-004l-4911000000-d40ba45835d1ee1b6c41 | 2017-09-12 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0k9f-4595000000-89ccbf45dbf9f474bd2f | 2017-09-01 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-007c-3249000000-3570f5d248c0ee0716ee | 2017-10-06 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | 2021-11-05 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_3) - 70eV, Positive | Not Available | 2021-11-05 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | 2021-11-05 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | 2021-11-05 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_3) - 70eV, Positive | Not Available | 2021-11-05 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-001i-0009000000-a157e4b4aa18bdbc2531 | 2012-07-24 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-052b-5900000000-42494c0ef4b45cfa5cf4 | 2012-07-24 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-052b-9700000000-d8a942a3dfdb061bd5c3 | 2012-07-24 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - EI-B (HITACHI M-80) , Positive | splash10-002o-9651000000-b0c55e50343bf8ab3077 | 2012-08-31 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-0a4j-4930000000-c5056c0aac78a148ea92 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 45V, Positive | splash10-052b-5910000000-fcf792a46552ed6e0028 | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 60V, Positive | splash10-052b-6900000000-6970a665800f041e9612 | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 30V, Positive | splash10-0532-6966000000-f57233040f10efef4ba9 | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 15V, Positive | splash10-001i-0009000000-0cff8a9a4549636b3f42 | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 75V, Positive | splash10-0a4j-9800000000-3aaefadc8d2e4ad3a522 | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 90V, Positive | splash10-0a6s-9600000000-17d56b65d46a46d9f653 | 2021-09-20 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0029000000-0af1b9a61aa5a9d514a4 | 2017-09-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0lzi-0295000000-0510776f8fa0d9ddcb6a | 2017-09-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00li-3970000000-1655d52be304b45389ae | 2017-09-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0009000000-bc250df2e599669d113e | 2017-09-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-002r-0097000000-41db31593b4f58cd4bc4 | 2017-09-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0bti-0092000000-464375e5c4a93d5d20b3 | 2017-09-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01q9-0019000000-bf0f51dc62b5855371a3 | 2021-09-22 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-0922000000-6997f50bd7502e7518f2 | 2021-09-22 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0cdi-3910000000-051d81ed4aaf877bebaf | 2021-09-22 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0039000000-8b733a2d2a661578ddbe | 2021-09-23 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004r-0049000000-1387b7ed039fc5504c87 | 2021-09-23 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00kr-0091000000-dc494a2f8f424f99a574 | 2021-09-23 | View Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-002f-9731000000-48d2266d41955ffa9ce4 | 2014-09-20 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 500 MHz, CDCl3, experimental) | Not Available | 2012-12-04 | View Spectrum | | 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, CDCl3, experimental) | Not Available | 2012-12-05 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | This is an endogenously produced metabolite found in the human body. It is used in metabolic reactions, catabolic reactions or waste generation. |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB00374 |
|---|
| PubChem Compound ID | 6238 |
|---|
| ChEMBL ID | CHEMBL1062 |
|---|
| ChemSpider ID | 6002 |
|---|
| KEGG ID | C01176 |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | 17252 |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Not Available |
|---|
| PDB ID | 3QZ |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | 17-Hydroxyprogesterone |
|---|
| References |
|---|
| Synthesis Reference | Schneider, Carlos; Silva, Mario; Zunza, Hilda; Becerra, Jose. Synthesis of 17.alpha.-hydroxyprogesterone from androstenedione. Boletin de la Sociedad Chilena de Quimica (1999), 44(2), 167-172. |
|---|
| MSDS | Link |
|---|
| General References | - Sahin Y, Kelestimur F: 17-Hydroxyprogesterone responses to gonadotrophin-releasing hormone agonist buserelin and adrenocorticotrophin in polycystic ovary syndrome: investigation of adrenal and ovarian cytochrome P450c17alpha dysregulation. Hum Reprod. 1997 May;12(5):910-3. [9194638 ]
- Kirchengast S, Hartmann B, Huber J: Serum levels of sex hormones, thyroid hormones, growth hormone, IGF I, and cortisol and their relations to body fat distribution in healthy women dependent on their menopausal status. Z Morphol Anthropol. 1996 Sep;81(2):223-34. [9270338 ]
- Hampl R, Lachman M, Novak Z, Sulcova J, Starka L: Serum levels of steroid hormones in men with varicocele and oligospermia as compared to normozoospermic men. Exp Clin Endocrinol. 1992;100(3):117-9. [1305061 ]
- Gibney MJ, Walsh M, Brennan L, Roche HM, German B, van Ommen B: Metabolomics in human nutrition: opportunities and challenges. Am J Clin Nutr. 2005 Sep;82(3):497-503. [16155259 ]
- White PC, Tusie-Luna MT, New MI, Speiser PW: Mutations in steroid 21-hydroxylase (CYP21). Hum Mutat. 1994;3(4):373-8. [8081391 ]
- Ibanez L, de Zegher F, Potau N: Anovulation after precocious pubarche: early markers and time course in adolescence. J Clin Endocrinol Metab. 1999 Aug;84(8):2691-5. [10443661 ]
- Katagiri M, Kagawa N, Waterman MR: The role of cytochrome b5 in the biosynthesis of androgens by human P450c17. Arch Biochem Biophys. 1995 Mar 10;317(2):343-7. [7893148 ]
- al Saedi S, Dean H, Dent W, Cronin C: Reference ranges for serum cortisol and 17-hydroxyprogesterone levels in preterm infants. J Pediatr. 1995 Jun;126(6):985-7. [7776113 ]
- Gupta MK, Guryev OL, Auchus RJ: 5alpha-reduced C21 steroids are substrates for human cytochrome P450c17. Arch Biochem Biophys. 2003 Oct 15;418(2):151-60. [14522586 ]
- Escobar-Morreale HF, San Millan JL, Smith RR, Sancho J, Witchel SF: The presence of the 21-hydroxylase deficiency carrier status in hirsute women: phenotype-genotype correlations. Fertil Steril. 1999 Oct;72(4):629-38. [10521100 ]
- Wilson SC, Hodgins MB, Scott JS: Incomplete masculinization due to a deficiency of 17 beta-hydroxysteroid dehydrogenase: comparison of prepubertal and peripubertal siblings. Clin Endocrinol (Oxf). 1987 Apr;26(4):459-69. [2820622 ]
- Toscano V, Sancesario G, Bianchi P, Cicardi C, Casilli D, Giacomini P: Cerebrospinal fluid estrone in pseudotumor cerebri: a change in cerebral steroid hormone metabolism? J Endocrinol Invest. 1991 Feb;14(2):81-6. [2061573 ]
- Mellon SH, Miller WL: Extraadrenal steroid 21-hydroxylation is not mediated by P450c21. J Clin Invest. 1989 Nov;84(5):1497-502. [2808702 ]
- Shackleton C, Malunowicz E: Apparent pregnene hydroxylation deficiency (APHD): seeking the parentage of an orphan metabolome. Steroids. 2003 Oct;68(9):707-17. [14625002 ]
- Bolt RJ, Van Weissenbruch MM, Popp-Snijders C, Sweep FG, Lafeber HN, Delemarre-van de Waal HA: Maturity of the adrenal cortex in very preterm infants is related to gestational age. Pediatr Res. 2002 Sep;52(3):405-10. [12193676 ]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|