| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2014-09-08 02:41:17 UTC |
|---|
| Update Date | 2014-12-24 20:26:54 UTC |
|---|
| Accession Number | T3D4655 |
|---|
| Identification |
|---|
| Common Name | Chloropicrin |
|---|
| Class | Small Molecule |
|---|
| Description | Chloropicrin, also known as PS, is a chemical compound currently used as a broad-spectrum antimicrobial, fungicide, herbicide, insecticide, and nematicide. Chloropicrin can be absorbed systemically through inhalation, ingestion, and the skin. At high concentrations it is severely irritating to the lungs, eyes, and skin. In World War I German forces used concentrated chloropicrin against Allied forces as a tear gas. While not as lethal as other chemical weapons, it caused vomiting and forced Allied soldiers to remove their masks to vomit, exposing them to other, more toxic chemical gases used as weapons during the war. (Wikipedia) |
|---|
| Compound Type | - Fungicide
- Lachrymator
- Organic Compound
- Organochloride
- Pesticide
- Synthetic Compound
|
|---|
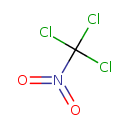
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | 1,1,1-Trichloronitromethane | | Chlorpikrin | | Trichlornitromethan | | Trichloronitromethane |
|
|---|
| Chemical Formula | CCl3NO2 |
|---|
| Average Molecular Mass | 164.375 g/mol |
|---|
| Monoisotopic Mass | 162.899 g/mol |
|---|
| CAS Registry Number | 76-06-2 |
|---|
| IUPAC Name | trichloro(nitro)methane |
|---|
| Traditional Name | chloropicrin |
|---|
| SMILES | ClC(Cl)(Cl)N(=O)=O |
|---|
| InChI Identifier | InChI=1S/CCl3NO2/c2-1(3,4)5(6)7 |
|---|
| InChI Key | InChIKey=LFHISGNCFUNFFM-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as trihalomethanes. These are organic compounds in which exactly three of the four hydrogen atoms of methane (CH4) are replaced by halogen atoms. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organohalogen compounds |
|---|
| Class | Alkyl halides |
|---|
| Sub Class | Halomethanes |
|---|
| Direct Parent | Trihalomethanes |
|---|
| Alternative Parents | |
|---|
| Substituents | - C-nitro compound
- Trihalomethane
- Organic nitro compound
- Organic oxoazanium
- Allyl-type 1,3-dipolar organic compound
- Propargyl-type 1,3-dipolar organic compound
- Organic 1,3-dipolar compound
- Alkyl chloride
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Organonitrogen compound
- Organochloride
- Organic oxygen compound
- Organic nitrogen compound
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | - Cytosol
- Endoplasmic reticulum
- Membrane
- Microsome
|
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Liquid |
|---|
| Appearance | Colorless liquid |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | -69°C | | Boiling Point | 112°C (dec) | | Solubility | Not Available | | LogP | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-02t9-1900000000-b880bd911a7b1c3641bd | 2017-09-20 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-2900000000-b87302c8573f1a84d7ef | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-0900000000-490e14dcba0ab27abbb7 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0002-9800000000-3bd82ea05629ac1f4c59 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0900000000-2f6b786f0387e1a5d1cb | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-1900000000-1186710e20df73f207cc | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014i-2900000000-0b318d856df2dfe79f85 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0900000000-d1165238c528e9ffc958 | 2021-09-25 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-0900000000-d1165238c528e9ffc958 | 2021-09-25 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03xr-0900000000-7e7b4cf50bc251d9a869 | 2021-09-25 | View Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-0159-7900000000-67b245e8df3a0c1c871e | 2014-09-20 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| PubChem Compound ID | 6423 |
|---|
| ChEMBL ID | CHEMBL1327143 |
|---|
| ChemSpider ID | 13861343 |
|---|
| KEGG ID | C18445 |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | 39285 |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Chloropicrin |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | T3D4655.pdf |
|---|
| General References | - Bessac BF, Jordt SE: Sensory detection and responses to toxic gases: mechanisms, health effects, and countermeasures. Proc Am Thorac Soc. 2010 Jul;7(4):269-77. doi: 10.1513/pats.201001-004SM. [20601631 ]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|