| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2014-09-11 05:13:46 UTC |
|---|
| Update Date | 2014-12-24 20:26:56 UTC |
|---|
| Accession Number | T3D4730 |
|---|
| Identification |
|---|
| Common Name | Medroxyprogesterone Acetate |
|---|
| Class | Small Molecule |
|---|
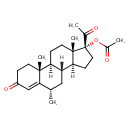
| Description | Medroxyprogesterone acetate (INN, USAN, BAN), also known as 17‘±-hydroxy-6‘±-methylprogesterone acetate, and commonly abbreviated as MPA, is a steroidal progestin, a synthetic variant of the human hormone progesterone. It is used as a contraceptive, in hormone replacement therapy and for the treatment of endometriosis as well as several other indications. MPA is a more potent derivative of its parent compound medroxyprogesterone (MP). While medroxyprogesterone is sometimes used as a synonym for medroxyprogesterone acetate, what is normally being administered is MPA and not MP. |
|---|
| Compound Type | - Antineoplastic Agent, Hormonal
- Contraceptive Agent
- Contraceptive Agent, Female
- Contraceptive Agent, Male
- Drug
- Ester
- Ether
- Organic Compound
- Synthetic Compound
|
|---|
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | (6alpha)-17-(Acetyloxy)-6-methylpreg-4-ene-3,20-dione | | 17-Acetoxy-6alpha-methylprogesterone | | 17-Acetoxy-6α-methylprogesterone | | 17alpha-Hydroxy-6alpha-methylprogesterone acetate | | 17α-hydroxy-6α-methylprogesterone acetate | | 6-alpha-Methyl-17-alpha-acetoxyprogesterone | | 6-alpha-Methyl-17-alpha-hydroxyprogesterone acetate | | 6alpha-Methyl-17-acetoxy progesterone | | 6alpha-Methyl-17alpha-hydroxyprogesterone acetate | | 6alpha-Methyl-4-pregnene-3,20-dion-17alpha-ol acetate | | 6α-Methyl-17-acetoxy progesterone | | 6α-Methyl-17α-hydroxyprogesterone acetate | | Depo-provera | | Depo-subq provera 104 | | Makena | | Medroxyacetate progesterone | | Medroxyprogesterone 17-acetate | | Medroxyprogesterone acetate | | Medroxyprogesterone acetic acid | | Methylacetoxyprogesterone | | Metigestrona | | MPA | | Provera |
|
|---|
| Chemical Formula | C24H34O4 |
|---|
| Average Molecular Mass | 386.524 g/mol |
|---|
| Monoisotopic Mass | 386.246 g/mol |
|---|
| CAS Registry Number | 71-58-9 |
|---|
| IUPAC Name | (1S,2R,8S,10R,11S,14R,15S)-14-acetyl-2,8,15-trimethyl-5-oxotetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadec-6-en-14-yl acetate |
|---|
| Traditional Name | provera |
|---|
| SMILES | [H][C@@]12CC[C@](OC(C)=O)(C(C)=O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])C[C@]([H])(C)C2=CC(=O)CC[C@]12C |
|---|
| InChI Identifier | InChI=1S/C24H34O4/c1-14-12-18-19(22(4)9-6-17(27)13-21(14)22)7-10-23(5)20(18)8-11-24(23,15(2)25)28-16(3)26/h13-14,18-20H,6-12H2,1-5H3/t14-,18+,19-,20-,22+,23-,24-/m0/s1 |
|---|
| InChI Key | InChIKey=PSGAAPLEWMOORI-PEINSRQWSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as gluco/mineralocorticoids, progestogins and derivatives. These are steroids with a structure based on a hydroxylated prostane moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Pregnane steroids |
|---|
| Direct Parent | Gluco/mineralocorticoids, progestogins and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Progestogin-skeleton
- Steroid ester
- 20-oxosteroid
- 3-oxo-delta-4-steroid
- 3-oxosteroid
- Oxosteroid
- Delta-4-steroid
- Cyclohexenone
- Alpha-acyloxy ketone
- Carboxylic acid ester
- Cyclic ketone
- Ketone
- Carboxylic acid derivative
- Monocarboxylic acid or derivatives
- Organic oxide
- Hydrocarbon derivative
- Organic oxygen compound
- Carbonyl group
- Organooxygen compound
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | 214.5°C | | Boiling Point | Not Available | | Solubility | 22.2mg/L | | LogP | 3.5 |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-qTof , Positive | splash10-00lu-0594000000-78579410f8f6799b400b | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-qTof , Positive | splash10-0a4l-3940000000-cf90a6f216d592e2ad4c | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-qTof , Positive | splash10-0udi-1339100000-e2ebb13d416a5b485ad5 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-002r-0449000000-c09cb0607e4a6118f05d | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-00dj-3940000000-2c9513d9a6a2092f5759 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-00di-3930000000-453ace561b4dc5712e85 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , positive | splash10-009i-2947000000-2c9190ea372fd54323e9 | 2017-09-14 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0009000000-05f1f54a7d2de1236908 | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-05p2-0049000000-ffc7818cd479acc19cab | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0uy0-0390000000-9ea2dbc66c9acf4b00ba | 2016-08-01 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000l-1009000000-0f7f31209fd83cf2cddf | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-2019000000-f92eb379c368e053c8ff | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a6u-8049000000-31808bd6c16e7dab93e3 | 2016-08-03 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 50.18 MHz, CDCl3, experimental) | Not Available | 2014-09-23 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Rapidly absorbed from GI tract |
|---|
| Mechanism of Toxicity | Progestins diffuse freely into target cells in the female reproductive tract, mammary gland, hypothalamus, and the pituitary and bind to the progesterone receptor. Once bound to the receptor, progestins slow the frequency of release of gonadotropin releasing hormone (GnRH) from the hypothalamus and blunt the pre-ovulatory LH surge. |
|---|
| Metabolism | Hepatic.
Route of Elimination: Following oral dosing, MPA is extensively metabolized in the liver via hydroxylation, with subsequent conjugation and elimination in the urine. Most MPA metabolites are excreted in the urine as glucuronide conjugates with only minor amounts excreted as sulfates.
Half Life: 50 days |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | 2B, possibly carcinogenic to humans. (3) |
|---|
| Uses/Sources | Used as a contraceptive and to treat secondary amenorrhea, abnormal uterine bleeding, pain associated with endometriosis, endometrial and renal cell carcinomas, paraphilia in males, GnRH-dependent forms of precocious puberty, as well as to prevent endometrial changes associated with estrogens. |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Side effects include loss of bone mineral density, BMD changes in adult women, bleeding irregularities, cancer risks, and thromboembolic disorders. |
|---|
| Treatment | Not Available |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB00603 |
|---|
| HMDB ID | Not Available |
|---|
| PubChem Compound ID | Not Available |
|---|
| ChEMBL ID | CHEMBL717 |
|---|
| ChemSpider ID | Not Available |
|---|
| KEGG ID | C08150 |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | 6715 |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Medroxyprogesterone |
|---|
| References |
|---|
| Synthesis Reference | Klaus ANNEN, Thomas Linz, Karl-Heinz Neff, Rolf Bohlmann, Henry Laurent, “PROCESS FOR PREPARING 17ALPHA-ACETOXY-6-METHYLENEPREGN-4-ENE-3,20-DIONE, MEDROXYPROGESTERONE ACETATE AND MEGESTROL ACETATE.” U.S. Patent US20090012321, issued January 08, 2009. |
|---|
| MSDS | T3D4730.pdf |
|---|
| General References | - Schindler AE, Campagnoli C, Druckmann R, Huber J, Pasqualini JR, Schweppe KW, Thijssen JH: Classification and pharmacology of progestins. Maturitas. 2008 Sep-Oct;61(1-2):171-80. [19434889 ]
- Lenco W, Mcknight M, Macdonald AS: Effects of cortisone acetate, methylprednisolone and medroxyprogesterone on wound contracture and epithelization in rabbits. Ann Surg. 1975 Jan;181(1):67-73. [1119869 ]
- International Agency for Research on Cancer (2014). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. [Link]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|