| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2014-09-11 05:16:24 UTC |
|---|
| Update Date | 2014-12-24 20:26:57 UTC |
|---|
| Accession Number | T3D4783 |
|---|
| Identification |
|---|
| Common Name | Nilutamide |
|---|
| Class | Small Molecule |
|---|
| Description | Nilutamide is an antineoplastic hormonal agent primarily used in the treatment of prostate cancer. Nilutamide is a pure, nonsteroidal anti-androgen with affinity for androgen receptors (but not for progestogen, estrogen, or glucocorticoid receptors). Consequently, Nilutamide blocks the action of androgens of adrenal and testicular origin that stimulate the growth of normal and malignant prostatic tissue. Prostate cancer is mostly androgen-dependent and can be treated with surgical or chemical castration. To date, antiandrogen monotherapy has not consistently been shown to be equivalent to castration. |
|---|
| Compound Type | - Amide
- Amine
- Androgen Antagonist
- Antineoplastic Agent
- Drug
- Metabolite
- Organic Compound
- Organofluoride
- Synthetic Compound
|
|---|
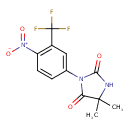
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | 5,5-Dimethyl-3-(alpha,alpha,alpha-trifluoro-4-nitro-m-tolyl)hydantoin | | Anandron | | Nilandron | | Nilutamida | | Nilutamidum |
|
|---|
| Chemical Formula | C12H10F3N3O4 |
|---|
| Average Molecular Mass | 317.221 g/mol |
|---|
| Monoisotopic Mass | 317.062 g/mol |
|---|
| CAS Registry Number | 63612-50-0 |
|---|
| IUPAC Name | 5,5-dimethyl-3-[4-nitro-3-(trifluoromethyl)phenyl]imidazolidine-2,4-dione |
|---|
| Traditional Name | nilutamide |
|---|
| SMILES | CC1(C)N=C(O)N(C1=O)C1=CC(=C(C=C1)N(=O)=O)C(F)(F)F |
|---|
| InChI Identifier | InChI=1S/C12H10F3N3O4/c1-11(2)9(19)17(10(20)16-11)6-3-4-8(18(21)22)7(5-6)12(13,14)15/h3-5H,1-2H3,(H,16,20) |
|---|
| InChI Key | InChIKey=XWXYUMMDTVBTOU-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as phenylhydantoins. These are heterocyclic aromatic compounds containing an imiazolidinedione moiety substituted by a phenyl group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Azolidines |
|---|
| Sub Class | Imidazolidines |
|---|
| Direct Parent | Phenylhydantoins |
|---|
| Alternative Parents | |
|---|
| Substituents | - 3-phenylhydantoin
- Phenylimidazolidine
- Alpha-amino acid or derivatives
- Trifluoromethylbenzene
- Nitrobenzene
- Nitroaromatic compound
- N-acyl urea
- Ureide
- Monocyclic benzene moiety
- Benzenoid
- Dicarboximide
- Organic nitro compound
- C-nitro compound
- Carbonic acid derivative
- Urea
- Azacycle
- Organic 1,3-dipolar compound
- Carboxylic acid derivative
- Propargyl-type 1,3-dipolar organic compound
- Allyl-type 1,3-dipolar organic compound
- Organic oxoazanium
- Carbonyl group
- Organohalogen compound
- Organic zwitterion
- Organic oxygen compound
- Hydrocarbon derivative
- Alkyl fluoride
- Organic nitrogen compound
- Organofluoride
- Organonitrogen compound
- Organooxygen compound
- Organopnictogen compound
- Organic oxide
- Alkyl halide
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | 4.19e-03 g/L | | LogP | 1.8 |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0a59-9051000000-b212093377487cffc158 | 2017-09-01 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 30V, Negative | splash10-0600-0094000000-0991ece9f9d42b94c643 | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 15V, Negative | splash10-014i-0019000000-7989a507a8031f03f612 | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 45V, Negative | splash10-00di-0090000000-1d63c70e06fcd4c6d922 | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 60V, Negative | splash10-0kmi-0390000000-05fc687a7f642e06e60a | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 60V, Positive | splash10-0kmi-0390000000-e2d42b542bf6dc07d5c0 | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 75V, Negative | splash10-0zfr-1970000000-f1091bfdddddc602902c | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 90V, Negative | splash10-0lz9-1920000000-14b04987997234218267 | 2021-09-20 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0009000000-f4acafe069c7fdcf8fc3 | 2016-08-02 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-9025000000-2c79c0bbfc87dd118bd7 | 2016-08-02 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4l-9000000000-a91f0e83aff94e2bad18 | 2016-08-02 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0009000000-19746ba07e5113297e15 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-4009000000-f2904499b324cc347c22 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4l-9000000000-740d06c64bdf821f7e3d | 2016-08-03 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Rapidly and completely absorbed, yielding high and persistent plasma concentrations. |
|---|
| Mechanism of Toxicity | Nilutamide competes with androgen for the binding of androgen receptors, consequently blocking the action of androgens of adrenal and testicular origin that stimulate the growth of normal and malignant prostatic tissue. This blockade of androgen receptors may result in growth arrest or transient tumor regression through inhibition of androgen-dependent DNA and protein synthesis. |
|---|
| Metabolism | The results of a human metabolism study using 14C-radiolabelled tablets show that nilutamide is extensively metabolized and less than 2% of the drug is excreted unchanged in urine after 5 days.
Route of Elimination: Nilutamide is extensively metabolized andless than 2% of the drug is excreted unchanged in urine after 5 days. Fecal elimination is negligible, ranging from 1.4% to 7% of the dose after 4 to 5 days.
Half Life: 38.0-59.1 hours |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | For use in combination with surgical castration for the treatment of metastatic prostate cancer involving distant lymph nodes, bone, or visceral organs (Stage D2). |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Symptoms of overdose include dizziness, general discomfort, headache, nausea, and vomiting. |
|---|
| Treatment | Not Available |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB00665 |
|---|
| HMDB ID | HMDB14803 |
|---|
| PubChem Compound ID | 4493 |
|---|
| ChEMBL ID | CHEMBL1274 |
|---|
| ChemSpider ID | 4337 |
|---|
| KEGG ID | C08164 |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | 7573 |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Nilutamide |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | - Kassouf W, Tanguay S, Aprikian AG: Nilutamide as second line hormone therapy for prostate cancer after androgen ablation fails. J Urol. 2003 May;169(5):1742-4. [12686822 ]
- Lukka H, Waldron T, Klotz L, Winquist E, Trachtenberg J: Maximal androgen blockade for the treatment of metastatic prostate cancer--a systematic review. Curr Oncol. 2006 Jun;13(3):81-93. [17576447 ]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|