| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2014-10-14 21:16:12 UTC |
|---|
| Update Date | 2014-12-24 20:27:01 UTC |
|---|
| Accession Number | T3D4976 |
|---|
| Identification |
|---|
| Common Name | Clomifene |
|---|
| Class | Small Molecule |
|---|
| Description | A triphenyl ethylene stilbene derivative which is an estrogen agonist or antagonist depending on the target tissue. [PubChem] |
|---|
| Compound Type | - Drug
- Estrogen Antagonist
- Fertility Agent, Female
- Metabolite
- Selective Estrogen Receptor Modulator
- Synthetic Compound
|
|---|
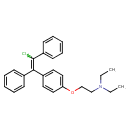
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | Androxal | | Chlomaphene | | Chloramifene | | Chloramiphene | | Cisclomiphene | | Clomid | | Clomifene citrate | | Clomifeno | | Clomifenum | | Clomifert | | Clomiphene | | Clomiphene Citrate | | Clomiphene citrate (Z,E) | | Clostilbegyt | | Omifin | | Racemic clomiphene citrate | | Serophene | | Zuclomiphene citrate |
|
|---|
| Chemical Formula | C26H28ClNO |
|---|
| Average Molecular Mass | 405.960 g/mol |
|---|
| Monoisotopic Mass | 405.186 g/mol |
|---|
| CAS Registry Number | 911-45-5 |
|---|
| IUPAC Name | {2-[4-(2-chloro-1,2-diphenylethenyl)phenoxy]ethyl}diethylamine |
|---|
| Traditional Name | pioner |
|---|
| SMILES | CCN(CC)CCOC1=CC=C(C=C1)C(=C(\Cl)C1=CC=CC=C1)\C1=CC=CC=C1 |
|---|
| InChI Identifier | InChI=1S/C26H28ClNO/c1-3-28(4-2)19-20-29-24-17-15-22(16-18-24)25(21-11-7-5-8-12-21)26(27)23-13-9-6-10-14-23/h5-18H,3-4,19-20H2,1-2H3/b26-25+ |
|---|
| InChI Key | InChIKey=GKIRPKYJQBWNGO-OCEACIFDSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as stilbenes. These are organic compounds containing a 1,2-diphenylethylene moiety. Stilbenes (C6-C2-C6 ) are derived from the common phenylpropene (C6-C3) skeleton building block. The introduction of one or more hydroxyl groups to a phenyl ring lead to stilbenoids. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Stilbenes |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Stilbenes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Stilbene
- Diphenylmethane
- Phenoxy compound
- Phenol ether
- Alkyl aryl ether
- Monocyclic benzene moiety
- Benzenoid
- Tertiary amine
- Tertiary aliphatic amine
- Ether
- Chloroalkene
- Haloalkene
- Vinyl halide
- Vinyl chloride
- Organooxygen compound
- Organonitrogen compound
- Organochloride
- Organohalogen compound
- Organic nitrogen compound
- Hydrocarbon derivative
- Amine
- Organic oxygen compound
- Organopnictogen compound
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | 116.5-118 °C | | Boiling Point | Not Available | | Solubility | Slightly soluble | | LogP | 7.2 |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-052r-9012000000-45df4fe468787edb6d89 | 2017-09-01 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-qTof , Positive | splash10-0a4i-0000900000-6e563271338e3449b9f0 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-0a4i-0000900000-6e563271338e3449b9f0 | 2017-09-14 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-1312900000-ca9aa2374db80c007b5f | 2017-07-26 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udr-4933200000-164fa87cdbe23cc72977 | 2017-07-26 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0fi9-9451000000-5927a010de880212b24f | 2017-07-26 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-1323900000-270e95f39bc9c23eae50 | 2017-07-26 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0pb9-4129400000-86f55d0b88e5983df5a4 | 2017-07-26 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-05i0-9454000000-848ee095f1842299cd00 | 2017-07-26 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0000900000-ff68a542dc5bb5928f03 | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0pb9-0801900000-ebdadab4e693385de318 | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0uk9-9710000000-e2c0a936a58120fde734 | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-1000900000-551503881b71c4f9f47c | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0ue9-7035900000-c45916b720c3b1c8bb5d | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-0019000000-2f31a2afd1fc50c9b823 | 2021-10-11 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Based on early studies with 14 C-labeled clomifene, the drug was shown to be readily absorbed orally in humans. |
|---|
| Mechanism of Toxicity | Clomifene has both estrogenic and anti-estrogenic properties, but its precise mechanism of action has not been determined. Clomifene appears to stumulate the release of gonadotropins, follicle-stimulating hormone (FSH), and leuteinizing hormone (LH), which leads to the development and maturation of ovarian follicle, ovulation, and subsequent development and function of the coprus luteum, thus resulting in pregnancy. Gonadotropin release may result from direct stimulation of the hypothalamic-pituitary axis or from a decreased inhibitory influence of estrogens on the hypothalamic-pituitary axis by competing with the endogenous estrogens of the uterus, pituitary, or hypothalamus. Clomifene has no apparent progestational, androgenic, or antrandrogenic effects and does not appear to interfere with pituitary-adrenal or pituitary-thyroid function. |
|---|
| Metabolism | Hepatic |
|---|
| Toxicity Values | The acute oral LD50 of clomifene is 1700 mg/kg in mice and 5750 mg/kg in rats. The toxic dose in humans is not known. Toxic effects accompanying acute overdosage of clomifene have not been reported. Signs and symptoms of overdosage as a result of the use of more than the recommended dose during clomifene therapy include nausea, vomiting, vasomotor flushes, visual blurring, spots or flashes, scotomata, ovarian enlargement with pelvic or abdominal pain. |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | 3, not classifiable as to its carcinogenicity to humans. (9) |
|---|
| Uses/Sources | Used mainly in female infertility due to anovulation (e.g. due to polycystic ovary syndrome) to induce ovulation. |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB00882 |
|---|
| HMDB ID | HMDB15020 |
|---|
| PubChem Compound ID | 1548953 |
|---|
| ChEMBL ID | CHEMBL1200667 |
|---|
| ChemSpider ID | 1265967 |
|---|
| KEGG ID | C06917 |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | 3752 |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | D002996 |
|---|
| Stitch ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Clomifene |
|---|
| References |
|---|
| Synthesis Reference | Allen, R.E., Palopoli, F.P., Schumann, E.L. and Van Carnpen, M.G. Jr.; US. Patent 2,914,563; November 24, 1959; assigned to The Wrn. S. Merrell Company. |
|---|
| MSDS | T3D4976.pdf |
|---|
| General References | - Purvin VA: Visual disturbance secondary to clomiphene citrate. Arch Ophthalmol. 1995 Apr;113(4):482-4. [7710399 ]
- Hayon T, Atlas L, Levy E, Dvilansky A, Shpilberg O, Nathan I: Multifactorial activities of nonsteroidal antiestrogens against leukemia. Cancer Detect Prev. 2003;27(5):389-96. [14585326 ]
- Fritz MA, Holmes RT, Keenan EJ: Effect of clomiphene citrate treatment on endometrial estrogen and progesterone receptor induction in women. Am J Obstet Gynecol. 1991 Jul;165(1):177-85. [1906682 ]
- Hughes E, Brown J, Collins JJ, Vanderkerchove P: Clomiphene citrate for unexplained subfertility in women. Cochrane Database Syst Rev. 2010 Jan 20;(1):CD000057. doi: 10.1002/14651858.CD000057.pub2. [20091498 ]
- Brown J, Farquhar C, Beck J, Boothroyd C, Hughes E: Clomiphene and anti-oestrogens for ovulation induction in PCOS. Cochrane Database Syst Rev. 2009 Oct 7;(4):CD002249. doi: 10.1002/14651858.CD002249.pub4. [19821295 ]
- Use of clomiphene citrate in women. Fertil Steril. 2006 Nov;86(5 Suppl 1):S187-93. [17055820 ]
- Homburg R: Oral agents for ovulation induction--clomiphene citrate versus aromatase inhibitors. Hum Fertil (Camb). 2008 Mar;11(1):17-22. doi: 10.1080/14647270701689670. [18320435 ]
- Homburg R: Clomiphene citrate--end of an era? A mini-review. Hum Reprod. 2005 Aug;20(8):2043-51. Epub 2005 May 5. [15878925 ]
- International Agency for Research on Cancer (2014). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. [Link]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|