| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2009-03-06 18:58:12 UTC |
|---|
| Update Date | 2014-12-24 20:21:15 UTC |
|---|
| Accession Number | T3D0163 |
|---|
| Identification |
|---|
| Common Name | 1,1,2-Trichloroethane |
|---|
| Class | Small Molecule |
|---|
| Description | 1,1,2-Trichloroethane is a colorless, sweet-smelling liquid that does not burn easily and boils at a higher temperature than water. It is used mostly where 1,1-dichloroethene (vinylidene chloride) is made. 1,1,2-Trichloroethane is used as a solvent. When it is released into the environment, most 1,1,2-trichloroethane finally ends up in the air, but some may enter groundwater. Breakdown in both the air and groundwater is slow. (5) |
|---|
| Compound Type | - Industrial/Workplace Toxin
- Lachrymator
- Organic Compound
- Organochloride
- Pollutant
- Solvent
- Synthetic Compound
|
|---|
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | 1,1, 2-Trichloroethane | | 1,1,2-Trichloraethan | | 1,1,2-Trichlorethane | | 1,2,2-Trichloroethane | | beta-T | | Beta-trichloroethane | | Beta.-trichloroethane | | Ethane trichloride | | Trichloroethane | | Vinyl trichloride | | Vinyltrichloride |
|
|---|
| Chemical Formula | C2H3Cl3 |
|---|
| Average Molecular Mass | 133.404 g/mol |
|---|
| Monoisotopic Mass | 131.930 g/mol |
|---|
| CAS Registry Number | 79-00-5 |
|---|
| IUPAC Name | 1,1,2-trichloroethane |
|---|
| Traditional Name | 1,1, 2-trichloroethane |
|---|
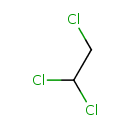
| SMILES | ClCC(Cl)Cl |
|---|
| InChI Identifier | InChI=1S/C2H3Cl3/c3-1-2(4)5/h2H,1H2 |
|---|
| InChI Key | InChIKey=UBOXGVDOUJQMTN-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as organochlorides. Organochlorides are compounds containing a chemical bond between a carbon atom and a chlorine atom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organohalogen compounds |
|---|
| Class | Organochlorides |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Organochlorides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Hydrocarbon derivative
- Organochloride
- Alkyl halide
- Alkyl chloride
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Liquid |
|---|
| Appearance | Colorless liquid. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | -36.6°C | | Boiling Point | 110 - 115 °C | | Solubility | 4.59 mg/mL at 25 °C [HORVATH,AL et al. (1999)] | | LogP | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-000t-9100000000-bf79232c76a22826ba5c | 2021-09-24 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-1900000000-3f6b6c4778619860fc19 | 2015-09-15 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001i-1900000000-de14959b0cb16e8cd707 | 2015-09-15 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01ot-9000000000-eece4c8a26c8a21d5fb7 | 2015-09-15 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-1900000000-cc11fb3585800aaf8723 | 2015-09-15 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-9400000000-bcf58e3a5f886b9be8cf | 2015-09-15 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9200000000-9058e4afd6f1129806b2 | 2015-09-15 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0900000000-6838b4c597e6d80ac4e2 | 2021-10-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-9000000000-c2fa753da65a4bac80a1 | 2021-10-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001i-7900000000-ff817bfd636e4a77346c | 2021-10-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0900000000-c8f6da92e011fc99d023 | 2021-10-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001i-0900000000-c8f6da92e011fc99d023 | 2021-10-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03dj-9000000000-97d9dd18a5aad6cec972 | 2021-10-12 | View Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-0002-9000000000-316c9aca46098f901713 | 2014-09-20 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 300 MHz, CDCl3, experimental) | Not Available | 2014-09-20 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 15.09 MHz, CDCl3, experimental) | Not Available | 2014-09-23 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | 2021-10-12 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | 2021-10-12 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | 2021-10-12 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | 2021-10-12 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | 2021-10-12 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | 2021-10-12 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | 2021-10-12 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | 2021-10-12 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | 2021-10-12 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | 2021-10-12 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | 2021-10-12 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | 2021-10-12 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | 2021-10-12 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | 2021-10-12 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | 2021-10-12 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | 2021-10-12 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | 2021-10-12 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | 2021-10-12 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | 2021-10-12 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | 2021-10-12 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Inhalation (6) ; oral (6) ; dermal (6) |
|---|
| Mechanism of Toxicity | Acyl chlorides and free radicals formed during the metabolism of 1,1,2-trichloroethane are reactive metabolites that can bind to proteins and nucleic acids (DNA, RNA), and are suspected of being cytotoxic, mutagenic, and carginogenic. (5, 1) |
|---|
| Metabolism | After 1,1,2-trichloroethane enters the body, it is carried by the blood to organs and tissues such as the liver, kidney, brain, heart, spleen, and fat. The primary metabolites identified are chloroacetic acid, S-carboxymethylcysteine, and thiodiacetic acid. S-carboxymethycysteine and thiodiacetic acid are formed from 1,1,2-trichloroethane following conjugation with glutathione. Chloroacetic acid is formed by hepatic cytochrome P-450. This reaction is thought to proceed via the acyl chloride. Cytochrome P-450 can also produce free radicals from 1,1,2-trichloroethane. Experiments show that most 1,1,2-trichloroethane leaves the body unchanged in the breath and as other substances that it is changed into in the urine in about 1 day. (5) |
|---|
| Toxicity Values | LD50: 837 mg/kg (Oral, Rat) (7)
LD50: 5.38 g/kg (Dermal, Rabbit) (7) |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | 3, not classifiable as to its carcinogenicity to humans. (4) |
|---|
| Uses/Sources | 1,1,2-Trichloroethane is used as a solvent. Exposure to 1,1,2-trichloroethane would most likely be from breathing vapors of the chemical or from skin contact. Drinking contaminated water is also a route of exposure. (5) |
|---|
| Minimum Risk Level | Acute Oral: 0.3 mg/kg/day (Mouse) (5)
Intermediate Oral: 0.04 mg/kg/day (Mouse) 9L422) |
|---|
| Health Effects | Inhalation of high levels of 1,1,2-trichloroethanein can affect the nervous system and cause sleepiness. 1,1,2-Trichloroethane may also affect the liver, kidney, and digestive tract, produce skin irritation, and affect the body's ability to fight infections. (5) |
|---|
| Symptoms | Inhalation or ingestion of 1,1,2-trichloroethane can cause dizziness, drowsiness, headache, nausea, shortness of breath, and unconsciousness. The compound can be absorbed following dermal contact. Skin exposure can also lead to temporary stinging and burning pain. Other symptoms of exposure to this compound may include irritation of the skin, eyes, nose, mucous membranes, and upper respiratory tract. (6, 2) |
|---|
| Treatment | Following ingestion, administer charcoal as a slurry (240 mL water/30 g charcoal). Usual dose: 25 to 100 g in adults/adolescents, 25 to 50 g in children (1 to 12 years), and 1 g/kg in infants less than 1 year old. Consider gastric lavage after ingestion of a potentially life-threatening amount of poison if it can be performed soon after ingestion. In case of seizures, administer a benzodiazepine IV. Following inhalation exposure, move patient to fresh air. Monitor for respiratory distress. If cough or difficulty breathing develops, evaluate for respiratory tract irritation, bronchitis, or pneumonitis. Administer oxygen and assist ventilation as required. Treat bronchospasm with inhaled beta2 agonist and oral or parenteral corticosteroids. In case of eye exposure, irrigate exposed eyes with copious amounts of room temperature water for at least 15 minutes. In case of dermal exposure, remove contaminated clothing and wash exposed area thoroughly with soap and water. (3) |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| PubChem Compound ID | 6574 |
|---|
| ChEMBL ID | CHEMBL43882 |
|---|
| ChemSpider ID | 6326 |
|---|
| KEGG ID | C19536 |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | 36018 |
|---|
| BioCyc ID | CPD-8985 |
|---|
| CTD ID | C024567 |
|---|
| Stitch ID | 1,1,2-Trichloroethane |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | 1412 |
|---|
| Wikipedia Link | 1,1,2-Trichloroethane |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | T3D0163.pdf |
|---|
| General References | - Mazzullo M, Colacci A, Grilli S, Prodi G, Arfellini G: 1,1,2-Trichloroethane: evidence of genotoxicity from short-term tests. Jpn J Cancer Res. 1986 Jun;77(6):532-9. [2426232 ]
- Bingham, E, Cohrssen, B, and Powell, CH (2001). Patty's Toxicology Volumes 1-9. 5th ed. New York, N.Y: John Wiley & Sons.
- Rumack BH (2009). POISINDEX(R) Information System. Englewood, CO: Micromedex, Inc. CCIS Volume 141, edition expires Aug, 2009.
- International Agency for Research on Cancer (2014). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. [Link]
- ATSDR - Agency for Toxic Substances and Disease Registry (1989). Toxicological profile for 1,1,2-trichloroethane. U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). [Link]
- International Programme on Chemical Safety (IPCS) INCHEM (1995). Poison Information Monograph for Cadmium. [Link]
- Organization for Economic Cooperation and Development (2000). Screening Information Data Set for 1,1,2-Trichloroethane (79-00-5). [Link]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|