| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2009-07-21 20:26:07 UTC |
|---|
| Update Date | 2014-12-24 20:25:49 UTC |
|---|
| Accession Number | T3D2690 |
|---|
| Identification |
|---|
| Common Name | Biotin |
|---|
| Class | Small Molecule |
|---|
| Description | Biotin is an enzyme co-factor present in minute amounts in every living cell. Biotin is also known as vitamin H or B7 or coenzyme R. It occurs mainly bound to proteins or polypeptides and is abundant in liver, kidney, pancreas, yeast, and milk. Biotin has been recognized as an essential nutrient. Our biotin requirement is fulfilled in part through diet, through endogenous reutilization of biotin and perhaps through capture of biotin generated in the intestinal flora. The utilization of biotin for covalent attachment to carboxylases and its reutilization through the release of carboxylase biotin after proteolytic degradation constitutes the 'biotin cycle'. Biotin deficiency is associated with neurological manifestations, skin rash, hair loss and metabolic disturbances that are thought to relate to the various carboxylase deficiencies (metabolic ketoacidosis with lactic acidosis). It has also been suggested that biotin deficiency is associated with protein malnutrition, and that marginal biotin deficiency in pregnant women may be teratogenic. Biotin acts as a carboxyl carrier in carboxylation reactions. There are four biotin-dependent carboxylases in mammals: those of propionyl-CoA (PCC), 3-methylcrotonyl-CoA (MCC), pyruvate (PC) and acetyl-CoA carboxylases (isoforms ACC-1 and ACC-2). All but ACC-2 are mitochondrial enzymes. The biotin moiety is covalently bound to the epsilon amino group of a Lysine residue in each of these carboxylases in a domain 60-80 amino acids long. The domain is structurally similar among carboxylases from bacteria to mammals. There are four biotin-dependent carboxylases in mammals: those of propionyl-CoA (PCC), 3-methylcrotonyl-CoA (MCC), pyruvate (PC) and acetyl-CoA carboxylases (isoforms ACC-1 and ACC-2). All but ACC-2 are mitochondrial enzymes. The biotin moiety is covalently bound to the epsilon amino group of a Lys residue in each of these carboxylases in a domain 60-80 amino acids long. The domain is structurally similar among carboxylases from bacteria to mammals. Evidence is emerging that biotin participates in processes other than classical carboxylation reactions. Specifically, novel roles for biotin in cell signaling, gene expression, and chromatin structure have been identified in recent years. Human cells accumulate biotin by using both the sodium-dependent multivitamin transporter and monocarboxylate transporter 1. These transporters and other biotin-binding proteins partition biotin to compartments involved in biotin signaling: cytoplasm, mitochondria, and nuclei. The activity of cell signals such as biotinyl-AMP, Sp1 and Sp3, nuclear factor (NF)-kappaB, and receptor tyrosine kinases depends on biotin supply. Consistent with a role for biotin and its catabolites in modulating these cell signals, greater than 2000 biotin-dependent genes have been identified in various human tissues. Many biotin-dependent gene products play roles in signal transduction and localize to the cell nucleus, consistent with a role for biotin in cell signaling. Posttranscriptional events related to ribosomal activity and protein folding may further contribute to effects of biotin on gene expression. Finally, research has shown that biotinidase and holocarboxylase synthetase mediate covalent binding of biotin to histones (DNA-binding proteins), affecting chromatin structure; at least seven biotinylation sites have been identified in human histones. Biotinylation of histones appears to play a role in cell proliferation, gene silencing, and the cellular response to DNA repair. Roles for biotin in cell signaling and chromatin structure are consistent with the notion that biotin has a unique significance in cell biology. (2, 3). |
|---|
| Compound Type | - Amine
- Animal Toxin
- Dietary Supplement
- Drug
- Ether
- Food Toxin
- Household Toxin
- Metabolite
- Micronutrient
- Natural Compound
- Nutraceutical
- Organic Compound
- Supplement
- Vitamin B Complex
|
|---|
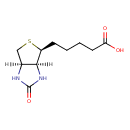
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | (+)-Biotin | | (+)-cis-Hexahydro-2-oxo-1H-thieno[3,4]imidazole-4-valerate | | (+)-cis-Hexahydro-2-oxo-1H-thieno[3,4]imidazole-4-valeric acid | | (3aS,4S,6aR)-Hexahydro-2-oxo-1H-thieno[3,4-D]imidazole-4-valerate | | (3aS,4S,6aR)-Hexahydro-2-oxo-1H-thieno[3,4-D]imidazole-4-valeric acid | | -(+)-biotin | | 1swk | | 1swn | | 1swr | | 5-(2-Oxohexahydro-1H-thieno[3,4-D]imidazol-4-yl)pentanoate | | 5-(2-Oxohexahydro-1H-thieno[3,4-D]imidazol-4-yl)pentanoic acid | | Appearex | | Biodermatin | | Bioepiderm | | Bios h | | Bios II | | Biotin Forte | | Biotina | | Biotine | | Biotinum | | cis-(+)-Tetrahydro-2-oxothieno[3,4]imidazoline-4-valerate | | cis-(+)-Tetrahydro-2-oxothieno[3,4]imidazoline-4-valeric acid | | cis-Hexahydro-2-oxo-1H-thieno(3,4)imidazole-4-valeric acid | | cis-Tetrahydro-2-oxothieno(3,4-D)imidazoline-4-valeric acid | | Coenzyme R | | D(+)-Biotin | | D-(+)-Biotin | | D-Biotin | | D-Biotin factor S | | delta-(+)-Biotin | | delta-Biotin | | delta-Biotin factor S | | Factor S | | Factor S (vitamin) | | Hexahydro-2-oxo-1H-thieno(3,4-D)imidazole-4-pentanoate | | Hexahydro-2-oxo-1H-thieno(3,4-D)imidazole-4-pentanoic acid | | Hexahydro-2-oxo-[3aS-(3aa,4b,6aa)]-1H-Thieno[3,4-D]imidazole-4-pentanoate | | Hexahydro-2-oxo-[3aS-(3aa,4b,6aa)]-1H-Thieno[3,4-D]imidazole-4-pentanoic acid | | Hexahydro-2-oxo-[3as-(3alpha,4beta,6alpha)]-1H-Thieno[3,4-D]imidazole-4-pentanoate | | Hexahydro-2-oxo-[3as-(3alpha,4beta,6alpha)]-1H-Thieno[3,4-D]imidazole-4-pentanoic acid | | Lutavit H2 | | Meribin | | Nail-ex | | Rovimix H 2 | | Vitamin B7 | | Vitamin H | | Vitamin-h |
|
|---|
| Chemical Formula | C10H16N2O3S |
|---|
| Average Molecular Mass | 244.311 g/mol |
|---|
| Monoisotopic Mass | 244.088 g/mol |
|---|
| CAS Registry Number | 58-85-5 |
|---|
| IUPAC Name | 5-[(3aS,4S,6aR)-2-oxo-hexahydro-1H-thieno[3,4-d]imidazol-4-yl]pentanoic acid |
|---|
| Traditional Name | 5-[(3aS,4S,6aR)-2-oxo-hexahydrothieno[3,4-d]imidazol-4-yl]pentanoic acid |
|---|
| SMILES | [H][C@@]1(CCCCC(O)=O)SC[C@]2([H])N=C(O)N[C@]12[H] |
|---|
| InChI Identifier | InChI=1S/C10H16N2O3S/c13-8(14)4-2-1-3-7-9-6(5-16-7)11-10(15)12-9/h6-7,9H,1-5H2,(H,13,14)(H2,11,12,15)/t6-,7-,9-/m0/s1 |
|---|
| InChI Key | InChIKey=YBJHBAHKTGYVGT-ZKWXMUAHSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as biotin and derivatives. These are organic compounds containing a ureido (tetrahydroimidizalone) ring fused with a tetrahydrothiophene ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Biotin and derivatives |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Biotin and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Biotin
- Imidazolyl carboxylic acid derivative
- Medium-chain fatty acid
- Heterocyclic fatty acid
- Thia fatty acid
- Fatty acid
- Fatty acyl
- Thiolane
- 2-imidazoline
- Isourea
- Azacycle
- Dialkylthioether
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Carboximidamide
- Carboxylic acid derivative
- Thioether
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Organic nitrogen compound
- Organonitrogen compound
- Organopnictogen compound
- Organooxygen compound
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Carbonyl group
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Endogenous |
|---|
| Cellular Locations | - Cytoplasm
- Extracellular
- Mitochondria
- Nucleus
|
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | |
|---|
| Pathways | | Name | SMPDB Link | KEGG Link |
|---|
| Biotin Metabolism | SMP00066 | map00780 | | Phosphoenolpyruvate carboxykinase deficiency 1 (PEPCK1) | SMP00560 | Not Available | | Fructose-1,6-diphosphatase deficiency | SMP00562 | Not Available | | Glycogen Storage Disease Type 1A (GSD1A) or Von Gierke Disease | SMP00374 | Not Available | | 2-Hydroxyglutric Aciduria (D And L Form) | SMP00136 | Not Available | | Threonine and 2-Oxobutanoate Degradation | SMP00452 | Not Available | | 2-Methyl-3-Hydroxybutryl CoA Dehydrogenase Deficiency | SMP00137 | Not Available | | 2-ketoglutarate dehydrogenase complex deficiency | SMP00549 | Not Available | | 3-Methylglutaconic Aciduria Type IV | SMP00141 | Not Available | | 3-Hydroxy-3-Methylglutaryl-CoA Lyase Deficiency | SMP00138 | Not Available | | 3-Methylcrotonyl Coa Carboxylase Deficiency Type I | SMP00237 | Not Available | | 3-Methylglutaconic Aciduria Type I | SMP00139 | Not Available | | 3-Methylglutaconic Aciduria Type III | SMP00140 | Not Available | | 3-hydroxyisobutyric acid dehydrogenase deficiency | SMP00521 | Not Available | | 3-hydroxyisobutyric aciduria | SMP00522 | Not Available | | 4-Hydroxybutyric Aciduria/Succinic Semialdehyde Dehydrogenase Deficiency | SMP00243 | Not Available | | Transfer of Acetyl Groups into Mitochondria | SMP00466 | Not Available | | Citric Acid Cycle | SMP00057 | map00020 | | Fatty Acid Biosynthesis | SMP00456 | map00061 | | Lactic Acidemia | SMP00313 | Not Available | | Propionic Acidemia | SMP00236 | Not Available | | Isovaleric acidemia | SMP00524 | Not Available | | Congenital lactic acidosis | SMP00546 | Not Available | | Methylmalonic Aciduria Due to Cobalamin-Related Disorders | SMP00201 | Not Available | | Malonic Aciduria | SMP00198 | Not Available | | Isovaleric Aciduria | SMP00238 | Not Available | | Methylmalonic Aciduria | SMP00200 | Not Available | | Alanine Metabolism | SMP00055 | map00250 | | Ammonia Recycling | SMP00009 | map00910 | | Beta-Ketothiolase Deficiency | SMP00173 | Not Available | | Biotinidase Deficiency | SMP00174 | Not Available | | Pyruvate Carboxylase Deficiency | SMP00350 | Not Available | | Multiple carboxylase deficiency, neonatal or early onset form | SMP00564 | Not Available | | Isobutyryl-coa dehydrogenase deficiency | SMP00523 | Not Available | | Malonyl-coa decarboxylase deficiency | SMP00502 | Not Available | | Pyruvate Dehydrogenase Complex Deficiency | SMP00212 | Not Available | | Mitochondrial complex II deficiency | SMP00548 | Not Available | | Pyruvate Decarboxylase E1 Component Deficiency (PDHE1 Deficiency) | SMP00334 | Not Available | | Methylmalonate Semialdehyde Dehydrogenase Deficiency | SMP00384 | Not Available | | Fumarase deficiency | SMP00547 | Not Available | | Pyruvate dehydrogenase deficiency (E3) | SMP00550 | Not Available | | Pyruvate dehydrogenase deficiency (E2) | SMP00551 | Not Available | | Pyruvate kinase deficiency | SMP00559 | Not Available | | Succinic semialdehyde dehydrogenase deficiency | SMP00567 | Not Available | | Valine, Leucine and Isoleucine Degradation | SMP00032 | map00280 | | Maple Syrup Urine Disease | SMP00199 | Not Available | | Glycogenosis, Type IA. Von gierke disease | SMP00581 | Not Available | | Gluconeogenesis | SMP00128 | Not Available | | Glutamate Metabolism | SMP00072 | map00250 | | Glycogenosis, Type IB | SMP00573 | Not Available | | Glycogenosis, Type IC | SMP00574 | Not Available | | Homocarnosinosis | SMP00385 | Not Available | | Hyperinsulinism-Hyperammonemia Syndrome | SMP00339 | Not Available | | Primary Hyperoxaluria Type I | SMP00352 | Not Available | | Primary hyperoxaluria II, PH2 | SMP00558 | Not Available | | Triosephosphate isomerase | SMP00563 | Not Available | | Leigh Syndrome | SMP00196 | Not Available | | Pyruvate Metabolism | SMP00060 | map00620 | | Propanoate Metabolism | SMP00016 | map00640 |
|
|---|
| Applications | |
|---|
| Biological Roles | |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | 232 dec°C | | Boiling Point | Not Available | | Solubility | 220 mg/L (at 25°C) | | LogP | 0.5 |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (Non-derivatized) | splash10-0f76-1960000000-b21ddd69490cac3254f8 | 2014-06-16 | View Spectrum | | GC-MS | GC-MS Spectrum - GC-MS (3 TMS) | splash10-0f76-3960000000-a8a94e2de123f66979d8 | 2014-06-16 | View Spectrum | | GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-0f76-1960000000-b21ddd69490cac3254f8 | 2017-09-12 | View Spectrum | | GC-MS | GC-MS Spectrum - GC-MS (Non-derivatized) | splash10-0f76-3960000000-a8a94e2de123f66979d8 | 2017-09-12 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0006-6910000000-11bfe0a5a77f7dfaa8c5 | 2016-09-22 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-00dl-9680000000-eb01d8147a82f7982b54 | 2017-10-06 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | 2021-11-05 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_3) - 70eV, Positive | Not Available | 2021-11-05 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | 2021-11-05 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | 2021-11-05 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_3) - 70eV, Positive | Not Available | 2021-11-05 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 10V, Negative | splash10-0006-0090000000-6d956bb533d353d449c9 | 2012-08-31 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 20V, Negative | splash10-0006-0190000000-01f67d1bdf8c742e48c8 | 2012-08-31 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 30V, Negative | splash10-0fxx-3920000000-f0b9613cbd9371e4be92 | 2012-08-31 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 40V, Negative | splash10-0006-9400000000-107f2a44f521c2513578 | 2012-08-31 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 50V, Negative | splash10-0006-9000000000-1a3f65d909dc40055e87 | 2012-08-31 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-IT (LC/MSD Trap XCT, Agilent Technologies) , Positive | splash10-004i-0090000000-c928e8d0a18f3f848262 | 2012-08-31 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-IT (LC/MSD Trap XCT, Agilent Technologies) , Positive | splash10-066s-1920000000-5f795e0b7f1d7cf986e5 | 2012-08-31 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-IT (LC/MSD Trap XCT, Agilent Technologies) , Positive | splash10-0ar1-1920000000-c298be862857cb3bbc7f | 2012-08-31 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-IT (LC/MSD Trap XCT, Agilent Technologies) , Positive | splash10-000t-0900000000-cdc4a4c359ff765fd32d | 2012-08-31 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-qTof , Positive | splash10-05xs-1920000000-3be430b63e9c748c681a | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , negative | splash10-0006-0090000000-bcffb0dcf77e8fd727a4 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , negative | splash10-0f6x-0390000000-c209523d36e9a7681f44 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , negative | splash10-0006-0090000000-c0f81ee86772310db415 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , negative | splash10-0006-0190000000-016eb89528e564c74720 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , negative | splash10-0fxx-3920000000-f0b9613cbd9371e4be92 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , negative | splash10-0006-9400000000-107f2a44f521c2513578 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , negative | splash10-0006-9000000000-d08e1b3709844e1e91b0 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , negative | splash10-0006-0190000000-21d0f2512788276bbb89 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Linear Ion Trap , negative | splash10-0udj-0690000000-dc372934024e58bdcc60 | 2017-09-14 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-002b-0290000000-43932f104dea28cbdfb8 | 2016-09-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-1960000000-d3673c6bc624b97f35be | 2016-09-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-052f-9600000000-00a4e152d89024a9b423 | 2016-09-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-1390000000-3f4b512cfa57b894ff94 | 2016-09-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0f6y-7890000000-ea1e647fc5b31e42af0b | 2016-09-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9100000000-e139c928dcc46b225bdb | 2016-09-12 | View Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-01pt-9500000000-a1e1ec56cf32236ac6b1 | 2018-05-25 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 600 MHz, H2O, experimental) | Not Available | 2012-12-04 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, D2O, experimental) | Not Available | 2016-10-22 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, D2O, experimental) | Not Available | 2016-10-22 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 100 MHz, H2O, predicted) | Not Available | 2022-08-20 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 100 MHz, H2O, predicted) | Not Available | 2022-08-20 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, H2O, predicted) | Not Available | 2022-08-20 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, H2O, predicted) | Not Available | 2022-08-20 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 200 MHz, H2O, predicted) | Not Available | 2022-08-20 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 200 MHz, H2O, predicted) | Not Available | 2022-08-20 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 300 MHz, H2O, predicted) | Not Available | 2022-08-20 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 300 MHz, H2O, predicted) | Not Available | 2022-08-20 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 400 MHz, H2O, predicted) | Not Available | 2022-08-20 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 400 MHz, H2O, predicted) | Not Available | 2022-08-20 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 500 MHz, H2O, predicted) | Not Available | 2022-08-20 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 500 MHz, H2O, predicted) | Not Available | 2022-08-20 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 600 MHz, H2O, predicted) | Not Available | 2022-08-20 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 600 MHz, H2O, predicted) | Not Available | 2022-08-20 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 700 MHz, H2O, predicted) | Not Available | 2022-08-20 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 700 MHz, H2O, predicted) | Not Available | 2022-08-20 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 800 MHz, H2O, predicted) | Not Available | 2022-08-20 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 800 MHz, H2O, predicted) | Not Available | 2022-08-20 | View Spectrum | | 1D NMR | 13C NMR Spectrum (1D, 900 MHz, H2O, predicted) | Not Available | 2022-08-20 | View Spectrum | | 1D NMR | 1H NMR Spectrum (1D, 900 MHz, H2O, predicted) | Not Available | 2022-08-20 | View Spectrum | | 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, 100%_DMSO, experimental) | Not Available | 2012-12-04 | View Spectrum | | 2D NMR | [1H, 1H]-TOCSY. Unexported temporarily by An Chi on Oct 15, 2021 until json or nmrML file is generated. 2D NMR Spectrum (experimental) | Not Available | 2012-12-04 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Oral.
Systemic - approximately 50% |
|---|
| Mechanism of Toxicity | Biotin is necessary for the proper functioning of enzymes that transport carboxyl units and fix carbon dioxide, and is required for various metabolic functions, including gluconeogenesis, lipogenesis, fatty acid biosynthesis, propionate metabolism, and catabolism of branched-chain amino acids. |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | For nutritional supplementation, also for treating dietary shortage or imbalance. |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Prolonged skin contact may cause irritation. |
|---|
| Treatment | Not Available |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB00121 |
|---|
| HMDB ID | HMDB00030 |
|---|
| PubChem Compound ID | 171548 |
|---|
| ChEMBL ID | CHEMBL857 |
|---|
| ChemSpider ID | 149962 |
|---|
| KEGG ID | C00120 |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | 104620 , 107970 , 135630 , 138320 , 142705 , 152445 , 164840 , 180640 , 186790 , 188370 , 188411 , 189918 , 189919 , 189920 , 200350 , 210200 , 210210 , 232000 , 232050 , 245400 , 253260 , 253270 , 266150 , 271930 , 275550 , 603032 , 604024 , 606054 , 606152 , 606557 , 606558 , 607483 , 608786 , 609010 , 609014 , 609018 , 609019 , 609751 , 610509 |
|---|
| ChEBI ID | 15956 |
|---|
| BioCyc ID | BIOTIN |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Biotin |
|---|
| PDB ID | BTN |
|---|
| ACToR ID | 2312 |
|---|
| Wikipedia Link | Biotin |
|---|
| References |
|---|
| Synthesis Reference | Takayoshi Mitsunaga, Kiyoto Chinushi, Tadashi Umezu, “Water-soluble biotin-containing preparation.” U.S. Patent US4277488, issued May, 1940. |
|---|
| MSDS | Link |
|---|
| General References | - Holmberg A, Blomstergren A, Nord O, Lukacs M, Lundeberg J, Uhlen M: The biotin-streptavidin interaction can be reversibly broken using water at elevated temperatures. Electrophoresis. 2005 Feb;26(3):501-10. [15690449 ]
- Gravel RA, Narang MA: Molecular genetics of biotin metabolism: old vitamin, new science. J Nutr Biochem. 2005 Jul;16(7):428-31. [15992684 ]
- Zempleni J: Uptake, localization, and noncarboxylase roles of biotin. Annu Rev Nutr. 2005;25:175-96. [16011464 ]
- Thuy LP, Belmont J, Nyhan WL: Prenatal diagnosis and treatment of holocarboxylase synthetase deficiency. Prenat Diagn. 1999 Feb;19(2):108-12. [10215065 ]
- Zempleni J, McCormick DB, Mock DM: Identification of biotin sulfone, bisnorbiotin methyl ketone, and tetranorbiotin-l-sulfoxide in human urine. Am J Clin Nutr. 1997 Feb;65(2):508-11. [9022537 ]
- Bussolati G, Gugliotta P, Volante M, Pace M, Papotti M: Retrieved endogenous biotin: a novel marker and a potential pitfall in diagnostic immunohistochemistry. Histopathology. 1997 Nov;31(5):400-7. [9416479 ]
- Mock DM, Stadler DD, Stratton SL, Mock NI: Biotin status assessed longitudinally in pregnant women. J Nutr. 1997 May;127(5):710-6. [9164991 ]
- Thuy LP, Sweetman L, Nyhan WL: A new immunochemical assay for biotin. Clin Chim Acta. 1991 Oct 31;202(3):191-7. [1814646 ]
- Limat A, Suormala T, Hunziker T, Waelti ER, Braathen LR, Baumgartner R: Proliferation and differentiation of cultured human follicular keratinocytes are not influenced by biotin. Arch Dermatol Res. 1996;288(1):31-8. [8750932 ]
- Bigham SL, Ballard JD, Giles KD, Clelland CS, Jeffcoat R, Griffin KS, Farley TD, Bushman DR, Wright JR: Synthesis and possible applications of biotin-linked copper clusters. Physiol Chem Phys Med NMR. 1990;22(2):63-72. [2100006 ]
- Mock DM, Stadler DD: Conflicting indicators of biotin status from a cross-sectional study of normal pregnancy. J Am Coll Nutr. 1997 Jun;16(3):252-7. [9176832 ]
- Bingham JP, Bian S, Tan ZY, Takacs Z, Moczydlowski E: Synthesis of a biotin derivative of iberiotoxin: binding interactions with streptavidin and the BK Ca2+-activated K+ channel expressed in a human cell line. Bioconjug Chem. 2006 May-Jun;17(3):689-99. [16704206 ]
- Mock DM: Biotin status: which are valid indicators and how do we know? J Nutr. 1999 Feb;129(2S Suppl):498S-503S. [10064317 ]
- Mock DM, Dyken ME: Biotin catabolism is accelerated in adults receiving long-term therapy with anticonvulsants. Neurology. 1997 Nov;49(5):1444-7. [9371938 ]
- Mock DM, Nyalala JO, Raguseo RM: A direct streptavidin-binding assay does not accurately quantitate biotin in human urine. J Nutr. 2001 Aug;131(8):2208-14. [11481419 ]
- Mardach R, Zempleni J, Wolf B, Cannon MJ, Jennings ML, Cress S, Boylan J, Roth S, Cederbaum S, Mock DM: Biotin dependency due to a defect in biotin transport. J Clin Invest. 2002 Jun;109(12):1617-23. [12070309 ]
- Mock DM, Heird GM: Urinary biotin analogs increase in humans during chronic supplementation: the analogs are biotin metabolites. Am J Physiol. 1997 Jan;272(1 Pt 1):E83-5. [9038855 ]
- Fujimoto W, Inaoki M, Fukui T, Inoue Y, Kuhara T: Biotin deficiency in an infant fed with amino acid formula. J Dermatol. 2005 Apr;32(4):256-61. [15863846 ]
- Schenker S, Hu ZQ, Johnson RF, Yang Y, Frosto T, Elliott BD, Henderson GI, Mock DM: Human placental biotin transport: normal characteristics and effect of ethanol. Alcohol Clin Exp Res. 1993 Jun;17(3):566-75. [8333586 ]
- Mock NI, Malik MI, Stumbo PJ, Bishop WP, Mock DM: Increased urinary excretion of 3-hydroxyisovaleric acid and decreased urinary excretion of biotin are sensitive early indicators of decreased biotin status in experimental biotin deficiency. Am J Clin Nutr. 1997 Apr;65(4):951-8. [9094878 ]
- Grafe F, Wohlrab W, Neubert RH, Brandsch M: Transport of biotin in human keratinocytes. J Invest Dermatol. 2003 Mar;120(3):428-33. [12603856 ]
- Sreekumar A, Poisson LM, Rajendiran TM, Khan AP, Cao Q, Yu J, Laxman B, Mehra R, Lonigro RJ, Li Y, Nyati MK, Ahsan A, Kalyana-Sundaram S, Han B, Cao X, Byun J, Omenn GS, Ghosh D, Pennathur S, Alexander DC, Berger A, Shuster JR, Wei JT, Varambally S, Beecher C, Chinnaiyan AM: Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature. 2009 Feb 12;457(7231):910-4. doi: 10.1038/nature07762. [19212411 ]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | | Gene | Gene Symbol | Gene ID | Interaction | Chromosome | Details |
|---|
|
|---|
| Down-Regulated Genes | | Gene | Gene Symbol | Gene ID | Interaction | Chromosome | Details |
|---|
|
|---|