| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2009-07-21 20:27:50 UTC |
|---|
| Update Date | 2014-12-24 20:25:53 UTC |
|---|
| Accession Number | T3D2914 |
|---|
| Identification |
|---|
| Common Name | Levorphanol |
|---|
| Class | Small Molecule |
|---|
| Description | Levorphanol is only found in individuals that have used or taken this drug. It is a narcotic analgesic that may be habit-forming. It is nearly as effective orally as by injection. [PubChem]Like other mu-agonist opioids it is believed to act at receptors in the periventricular and periaqueductal gray matter in both the brain and spinal cord to alter the transmission and perception of pain. |
|---|
| Compound Type | - Amine
- Analgesic, Opioid
- Drug
- Metabolite
- Narcotic
- Organic Compound
- Synthetic Compound
|
|---|
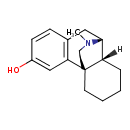
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | Aromarone | | Cetarin | | Dea No. 9220 | | Dea No. 9733 | | Lemoran | | Levo-Dromoran | | Levorfanol | | Levorfanolo | | Levorphan | | Levorphanal | | Lévorphanol | | Levorphanol Dl-Form | | Levorphanolum | | Methorphinan | | Racemethorphanum |
|
|---|
| Chemical Formula | C17H23NO |
|---|
| Average Molecular Mass | 257.371 g/mol |
|---|
| Monoisotopic Mass | 257.178 g/mol |
|---|
| CAS Registry Number | 77-07-6 |
|---|
| IUPAC Name | (1R,9R,10R)-17-methyl-17-azatetracyclo[7.5.3.0¹,¹⁰.0²,⁷]heptadeca-2(7),3,5-trien-4-ol |
|---|
| Traditional Name | levorphanol |
|---|
| SMILES | [H][C@@]12CC3=C(C=C(O)C=C3)[C@]3(CCCC[C@@]13[H])CCN2C |
|---|
| InChI Identifier | InChI=1S/C17H23NO/c1-18-9-8-17-7-3-2-4-14(17)16(18)10-12-5-6-13(19)11-15(12)17/h5-6,11,14,16,19H,2-4,7-10H2,1H3/t14-,16+,17+/m0/s1 |
|---|
| InChI Key | InChIKey=JAQUASYNZVUNQP-USXIJHARSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as morphinans. These are polycyclic compounds with a four-ring skeleton with three condensed six-member rings forming a partially hydrogenated phenanthrene moiety, one of which is aromatic while the two others are alicyclic. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Alkaloids and derivatives |
|---|
| Class | Morphinans |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Morphinans |
|---|
| Alternative Parents | |
|---|
| Substituents | - Morphinan
- Phenanthrene
- Benzazocine
- Tetralin
- 1-hydroxy-2-unsubstituted benzenoid
- Aralkylamine
- Piperidine
- Benzenoid
- Tertiary aliphatic amine
- Tertiary amine
- Organoheterocyclic compound
- Azacycle
- Organopnictogen compound
- Amine
- Organic oxygen compound
- Organooxygen compound
- Organonitrogen compound
- Organic nitrogen compound
- Hydrocarbon derivative
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | 198-199°C | | Boiling Point | Not Available | | Solubility | 1840 mg/L | | LogP | 3.11 |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00ou-0190000000-14189f3c821f5d9240bf | 2017-09-01 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-0hbi-2096000000-da9354b628843987f142 | 2017-10-06 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0090000000-6ba0fc98a8d249fe2da0 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-0090000000-d3e64fd4fd1d790a54bb | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0596-6790000000-7a77fc910d8d984d1012 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0090000000-503f808754310f636373 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-0090000000-bce69f729c40b2f3bed9 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-002g-1290000000-97ee80b268d9fa4de679 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0090000000-07569ae87a2b34cbffc0 | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-0090000000-02552f8621a9e610534e | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03di-2960000000-72882cb61c3a77dadafb | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0090000000-4d2203d29a4418e5d773 | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-0090000000-4d2203d29a4418e5d773 | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0f6t-0490000000-d5048ebe52cdbfced464 | 2021-10-11 | View Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-0a4i-5930000000-4593c62f1e2a0579a248 | 2014-09-20 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Subcutaneous, Intravenous, or Intramuscular injection ; Oral. Levorphanol is well absorbed after PO administration with peak plasma concentrations occurring approximately 1 hour after dosing. |
|---|
| Mechanism of Toxicity | Like other mu-agonist opioids it is believed to act at receptors in the periventricular and periaqueductal gray matter in both the brain and spinal cord to alter the transmission and perception of pain. |
|---|
| Metabolism | Levorphanol is extensively metabolized in the liver and is eliminated as the glucuronide metabolite.
Half Life: 11-16 hours |
|---|
| Toxicity Values | LD50: 150 mg/kg (Oral, Rat) (1) |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | For the management of moderate to severe pain or as a preoperative medication where an opioid analgesic is appropriate |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Medical problems can include congested lungs, liver disease, tetanus, infection of the heart valves, skin abscesses, anemia and pneumonia. Death can occur from overdose. |
|---|
| Symptoms | Signs of overdose include nausea, emesis, dizziness, respiratory depression, hypotension, urinary retention, cardiac arrhythmias, allergic reactions, skin rash, and uticaria. |
|---|
| Treatment | The specific treatment of suspected levorphanol tartrate overdosage is immediate establishment of an adequate airway and ventilation, followed (if necessary) by intravenous naloxone. The respiratory and cardiac status of the patient should be continuously monitored and appropriate supportive measures instituted, such as oxygen, intravenous fluids and/or vasopressors, if required. Physicians are reminded that the duration of levorphanol action far exceeds the duration of action of naloxone, and repeated dosing with naloxone may be required. Naloxone should be administered cautiously to persons known or suspected to be physically dependent on Levorphanol. In such cases an abrupt and complete reversal of opioid effects may precipitate an acute abstinence syndrome. If necessary to administer naloxone to the physically dependent patient, the antagonist should be administered with extreme care and by titration with smaller than usual doses of the antagonist. (3) |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB00854 |
|---|
| HMDB ID | HMDB14992 |
|---|
| PubChem Compound ID | 5359272 |
|---|
| ChEMBL ID | CHEMBL592 |
|---|
| ChemSpider ID | 16736212 |
|---|
| KEGG ID | C08014 |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | 119572 |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Levorphanol |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Levorphanol |
|---|
| References |
|---|
| Synthesis Reference | Joseph P. Haar, “Process for the Production of Levorphanol and Related Compounds.” U.S. Patent US20080146805, issued June 19, 2008. |
|---|
| MSDS | Link |
|---|
| General References | - Wishart DS, Knox C, Guo AC, Cheng D, Shrivastava S, Tzur D, Gautam B, Hassanali M: DrugBank: a knowledgebase for drugs, drug actions and drug targets. Nucleic Acids Res. 2008 Jan;36(Database issue):D901-6. Epub 2007 Nov 29. [18048412 ]
- Drugs.com [Link]
- RxList: The Internet Drug Index (2009). [Link]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|