Ondansetron (T3D2925)
| Record Information | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 2.0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Creation Date | 2009-07-21 20:27:55 UTC | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 2014-12-24 20:25:53 UTC | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Accession Number | T3D2925 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Common Name | Ondansetron | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Small Molecule | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | Ondansetron is a well tolerated drug with few side effects. Headache, constipation, and dizziness are the most commonly reported side effects associated with its use. There have been no significant drug interactions reported with this drugs use. It is broken down by the hepatic cytochrome P450 system and it has little effect on the metabolism of other drugs broken down by this system; Ondansetron is a serotonin 5-HT3 receptor antagonist used mainly to treat nausea and vomiting following chemotherapy. Its effects are thought to be on both peripheral and central nerves. One part is to reduce the activity of the vagus nerve, which is a nerve that activates the vomiting center in the medulla oblongata, the other is a blockage of serotonin receptors in the chemoreceptor trigger zone. It does not have much effect on vomiting due to motion sickness. This drug does not have any effect on dopamine receptors or muscarinic receptors; A competitive serotonin type 3 receptor antagonist. It is effective in the treatment of nausea and vomiting caused by cytotoxic chemotherapy drugs, including cisplatin, and has reported anxiolytic and neuroleptic properties; Ondansetron (INN) is a serotonin 5-HT3 receptor antagonist used mainly to treat nausea and vomiting following chemotherapy. Its effects are thought to be on both peripheral and central nerves. One part is to reduce the activity of the vagus nerve, which is a nerve that activates the vomiting center in the medulla oblongata, the other is a blockage of serotonin receptors in the chemoreceptor trigger zone. It does not have much effect on vomiting due to motion sickness. This drug does not have any effect on dopamine receptors or muscarinic receptors. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Compound Type |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
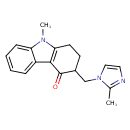
| Chemical Structure | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula | C18H19N3O | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Mass | 293.363 g/mol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Mass | 293.153 g/mol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS Registry Number | 99614-02-5 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name | 9-methyl-3-[(2-methyl-1H-imidazol-1-yl)methyl]-2,3,4,9-tetrahydro-1H-carbazol-4-one | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional Name | ondansetron | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES | CN1C2=C(C3=CC=CC=C13)C(=O)C(CN1C=CN=C1C)CC2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Identifier | InChI=1/C18H19N3O/c1-12-19-9-10-21(12)11-13-7-8-16-17(18(13)22)14-5-3-4-6-15(14)20(16)2/h3-6,9-10,13H,7-8,11H2,1-2H3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key | InChIKey=FELGMEQIXOGIFQ-UHFFFAOYNA-N | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | belongs to the class of organic compounds known as carbazoles. Carbazoles are compounds containing a three ring system containing a pyrrole ring fused on either side to a benzene ring. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organoheterocyclic compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Indoles and derivatives | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Carbazoles | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Carbazoles | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aromatic heteropolycyclic compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Status | Detected and Not Quantified | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Origin | Exogenous | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biofluid Locations | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Tissue Locations |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Applications | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Roles | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Roles | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State | Solid | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | White powder. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Toxicity Profile | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Route of Exposure | Ondansetron is well absorbed after oral administration and undergoes limited first-pass metabolism. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mechanism of Toxicity | Ondansetron is a selective serotonin 5-HT3 receptor antagonist. The antiemetic activity of the drug is brought about through the inhibition of 5-HT3 receptors present both centrally (medullary chemoreceptor zone) and peripherally (GI tract). This inhibition of 5-HT3 receptors in turn inhibits the visceral afferent stimulation of the vomiting center, likely indirectly at the level of the area postrema, as well as through direct inhibition of serotonin activity within the area postrema and the chemoreceptor trigger zone. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolism | Hepatic Half Life: 5.7 hours | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Toxicity Values | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Lethal Dose | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Uses/Sources | For the prevention of nausea and vomiting associated with emetogenic cancer chemotherapy, postoperation, and radiation. Also used for the treatment of postoperative nausea and vomiting. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Minimum Risk Level | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Health Effects | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symptoms | Low blood pressure and fainting, sudden blindness, severe constipation | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Treatment | There is no specific antidote for ondansetron overdose. Patients should be managed with appropriate supportive therapy. (8) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Normal Concentrations | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Abnormal Concentrations | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| DrugBank ID | DB00904 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| HMDB ID | HMDB05035 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PubChem Compound ID | 4595 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ChEMBL ID | CHEMBL46 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ChemSpider ID | 4434 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| KEGG ID | C07325 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| UniProt ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| OMIM ID | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ChEBI ID | 103183 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BioCyc ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CTD ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Stitch ID | Ondansetron | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PDB ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ACToR ID | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikipedia Link | Ondansetron | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference | Peter Bod, Kalman Harsanyi, Ferenc Trischler, Eva Fekecs, Attila Csehi, Bela Hegedus, Eva Mersich nee Donat, Gyorgyi Szabo nee Komlosi, Erika Horvath nee Sziki, “Process for preparing ondansetron.” U.S. Patent US5478949, issued September, 1990. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MSDS | Link | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| General References |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Gene Regulation | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Up-Regulated Genes | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Down-Regulated Genes | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Targets
- General Function:
- Voltage-gated potassium channel activity
- Specific Function:
- This is one of the several different receptors for 5-hydroxytryptamine (serotonin), a biogenic hormone that functions as a neurotransmitter, a hormone, and a mitogen. This receptor is a ligand-gated ion channel, which when activated causes fast, depolarizing responses in neurons. It is a cation-specific, but otherwise relatively nonselective, ion channel.
- Gene Name:
- HTR3A
- Uniprot ID:
- P46098
- Molecular Weight:
- 55279.835 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | 0.0028 uM | Not Available | BindingDB 50000493 |
| Inhibitory | 0.0034 uM | Not Available | BindingDB 50000493 |
| Inhibitory | 0.016 uM | Not Available | BindingDB 50000493 |
| IC50 | 0.00009 uM | Not Available | BindingDB 50000493 |
References
- Chen X, Ji ZL, Chen YZ: TTD: Therapeutic Target Database. Nucleic Acids Res. 2002 Jan 1;30(1):412-5. [11752352 ]
- Artaiz I, Zazpe A, Del Rio J: Characterization of serotonergic mechanisms involved in the behavioural inhibition induced by 5-hydroxytryptophan in a modified light-dark test in mice. Behav Pharmacol. 1998 Mar;9(2):103-12. [10065930 ]
- Fortuno A, Ballaz S, Del Rio J, Barber A: CCK-mediated response in the activation of 5-HT receptor types in the guinea-pig ileum. J Physiol Biochem. 1999 Jun;55(2):85-92. [10517265 ]
- Llacer JM, Gallardo V, Delgado R, Parraga J, Martin D, Ruiz MA: X-ray diffraction and electron microscopy in the polymorphism study of ondansetron hydrochloride. Drug Dev Ind Pharm. 2001 Oct;27(9):899-908. [11763467 ]
- Carvalho F, Macedo D, Bandeira I, Maldonado I, Salles L, Azevedo MF, Rocha MA Jr, Fregoneze JB, De Castro-e-Silva E: Central 5-HT3 receptor stimulation by m-CPBG increases blood glucose in rats. Horm Metab Res. 2002 Feb;34(2):55-61. [11972287 ]
- Arcioni R, della Rocca M, Romano S, Romano R, Pietropaoli P, Gasparetto A: Ondansetron inhibits the analgesic effects of tramadol: a possible 5-HT(3) spinal receptor involvement in acute pain in humans. Anesth Analg. 2002 Jun;94(6):1553-7, table of contents. [12032025 ]
- Bang-Andersen B, Ruhland T, Jorgensen M, Smith G, Frederiksen K, Jensen KG, Zhong H, Nielsen SM, Hogg S, Mork A, Stensbol TB: Discovery of 1-[2-(2,4-dimethylphenylsulfanyl)phenyl]piperazine (Lu AA21004): a novel multimodal compound for the treatment of major depressive disorder. J Med Chem. 2011 May 12;54(9):3206-21. doi: 10.1021/jm101459g. Epub 2011 Apr 12. [21486038 ]
- Yang Z, Fairfax DJ, Maeng JH, Masih L, Usyatinsky A, Hassler C, Isaacson S, Fitzpatrick K, DeOrazio RJ, Chen J, Harding JP, Isherwood M, Dobritsa S, Christensen KL, Wierschke JD, Bliss BI, Peterson LH, Beer CM, Cioffi C, Lynch M, Rennells WM, Richards JJ, Rust T, Khmelnitsky YL, Cohen ML, Manning DD: Discovery of 2-substituted benzoxazole carboxamides as 5-HT3 receptor antagonists. Bioorg Med Chem Lett. 2010 Nov 15;20(22):6538-41. doi: 10.1016/j.bmcl.2010.09.038. Epub 2010 Sep 16. [20889341 ]
- Rizzi JP, Nagel AA, Rosen T, McLean S, Seeger T: An initial three-component pharmacophore for specific serotonin-3 receptor ligands. J Med Chem. 1990 Oct;33(10):2721-5. [2145434 ]
- Rosen T, Nagel AA, Rizzi JP, Ives JL, Daffeh JB, Ganong AH, Guarino K, Heym J, McLean S, Nowakowski JT, et al.: Thiazole as a carbonyl bioisostere. A novel class of highly potent and selective 5-HT3 receptor antagonists. J Med Chem. 1990 Oct;33(10):2715-20. [2213824 ]
- Ramsook C, Sahagun-Carreon I, Kozinetz CA, Moro-Sutherland D: A randomized clinical trial comparing oral ondansetron with placebo in children with vomiting from acute gastroenteritis. Ann Emerg Med. 2002 Apr;39(4):397-403. [11919526 ]
- General Function:
- Voltage-gated potassium channel activity involved in ventricular cardiac muscle cell action potential repolarization
- Specific Function:
- Pore-forming (alpha) subunit of voltage-gated inwardly rectifying potassium channel. Channel properties are modulated by cAMP and subunit assembly. Mediates the rapidly activating component of the delayed rectifying potassium current in heart (IKr). Isoforms USO have no channel activity by themself, but modulates channel characteristics by forming heterotetramers with other isoforms which are retained intracellularly and undergo ubiquitin-dependent degradation.
- Gene Name:
- KCNH2
- Uniprot ID:
- Q12809
- Molecular Weight:
- 126653.52 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| IC50 | 0.4 uM | Not Available | BindingDB 50000493 |
| IC50 | 0.81283 uM | Not Available | BindingDB 50000493 |
| IC50 | 0.813 uM | Not Available | BindingDB 50000493 |
References
- Yang Z, Fairfax DJ, Maeng JH, Masih L, Usyatinsky A, Hassler C, Isaacson S, Fitzpatrick K, DeOrazio RJ, Chen J, Harding JP, Isherwood M, Dobritsa S, Christensen KL, Wierschke JD, Bliss BI, Peterson LH, Beer CM, Cioffi C, Lynch M, Rennells WM, Richards JJ, Rust T, Khmelnitsky YL, Cohen ML, Manning DD: Discovery of 2-substituted benzoxazole carboxamides as 5-HT3 receptor antagonists. Bioorg Med Chem Lett. 2010 Nov 15;20(22):6538-41. doi: 10.1016/j.bmcl.2010.09.038. Epub 2010 Sep 16. [20889341 ]
- Tobita M, Nishikawa T, Nagashima R: A discriminant model constructed by the support vector machine method for HERG potassium channel inhibitors. Bioorg Med Chem Lett. 2005 Jun 2;15(11):2886-90. [15911273 ]
- Jia L, Sun H: Support vector machines classification of hERG liabilities based on atom types. Bioorg Med Chem. 2008 Jun 1;16(11):6252-60. doi: 10.1016/j.bmc.2008.04.028. Epub 2008 Apr 16. [18448342 ]
- Keseru GM: Prediction of hERG potassium channel affinity by traditional and hologram qSAR methods. Bioorg Med Chem Lett. 2003 Aug 18;13(16):2773-5. [12873512 ]
3. Serotonin Receptors (Protein Group)
- General Function:
- Serotonin receptor activity
- Specific Function:
- G-protein coupled receptor for 5-hydroxytryptamine (serotonin). Also functions as a receptor for various drugs and psychoactive substances. Ligand binding causes a conformation change that triggers signaling via guanine nucleotide-binding proteins (G proteins) and modulates the activity of down-stream effectors, such as adenylate cyclase. Beta-arrestin family members inhibit signaling via G proteins and mediate activation of alternative signaling pathways. Signaling inhibits adenylate cyclase activity and activates a phosphatidylinositol-calcium second messenger system that regulates the release of Ca(2+) ions from intracellular stores. Plays a role in the regulation of 5-hydroxytryptamine release and in the regulation of dopamine and 5-hydroxytryptamine metabolism. Plays a role in the regulation of dopamine and 5-hydroxytryptamine levels in the brain, and thereby affects neural activity, mood and behavior. Plays a role in the response to anxiogenic stimuli.
- Included Proteins:
- P08908 , P28222 , P28221 , P28566 , P30939 , P28223 , P41595 , P28335 , P46098 , O95264 , Q8WXA8 , Q70Z44 , A5X5Y0 , Q13639 , P50406 , P34969
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | 0.0076 uM | Not Available | BindingDB 50000493 |
| Inhibitory | 0.012 uM | Not Available | BindingDB 50000493 |
| Dissociation | 0.0000794 uM | Not Available | BindingDB 50000493 |
| Dissociation | 0.000398 uM | Not Available | BindingDB 50000493 |
| Dissociation | 0.05012 uM | Not Available | BindingDB 50000493 |
References
- Hibert MF, Hoffmann R, Miller RC, Carr AA: Conformation-activity relationship study of 5-HT3 receptor antagonists and a definition of a model for this receptor site. J Med Chem. 1990 Jun;33(6):1594-600. [2342053 ]
- Kishibayashi N, Miwa Y, Hayashi H, Ishii A, Ichikawa S, Nonaka H, Yokoyama T, Suzuki F: 5-HT3 receptor antagonists. 3. Quinoline derivatives which may be effective in the therapy of irritable bowel syndrome. J Med Chem. 1993 Oct 29;36(22):3286-92. [8230119 ]
- Macor JE, Gurley D, Lanthorn T, Loch J, Mack RA, Mullen G, Tran O, Wright N, Gordon JC: The 5-HT3 antagonist tropisetron (ICS 205-930) is a potent and selective alpha7 nicotinic receptor partial agonist. Bioorg Med Chem Lett. 2001 Feb 12;11(3):319-21. [11212100 ]
- General Function:
- Monovalent cation:proton antiporter activity
- Specific Function:
- Solute transporter for tetraethylammonium (TEA), 1-methyl-4-phenylpyridinium (MPP), cimetidine, N-methylnicotinamide (NMN), metformin, creatinine, guanidine, procainamide, topotecan, estrone sulfate, acyclovir, ganciclovir and also the zwitterionic cephalosporin, cephalexin and cephradin. Seems to also play a role in the uptake of oxaliplatin (a new platinum anticancer agent). Able to transport paraquat (PQ or N,N-dimethyl-4-4'-bipiridinium); a widely used herbicid. Responsible for the secretion of cationic drugs across the brush border membranes.
- Gene Name:
- SLC47A1
- Uniprot ID:
- Q96FL8
- Molecular Weight:
- 61921.585 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| IC50 | 0.03 uM | Not Available | BindingDB 50000493 |
| IC50 | 0.15 uM | Not Available | BindingDB 50000493 |
| IC50 | 0.16 uM | Not Available | BindingDB 50000493 |
References
- Wittwer MB, Zur AA, Khuri N, Kido Y, Kosaka A, Zhang X, Morrissey KM, Sali A, Huang Y, Giacomini KM: Discovery of potent, selective multidrug and toxin extrusion transporter 1 (MATE1, SLC47A1) inhibitors through prescription drug profiling and computational modeling. J Med Chem. 2013 Feb 14;56(3):781-95. doi: 10.1021/jm301302s. Epub 2013 Jan 22. [23241029 ]
- Kido Y, Matsson P, Giacomini KM: Profiling of a prescription drug library for potential renal drug-drug interactions mediated by the organic cation transporter 2. J Med Chem. 2011 Jul 14;54(13):4548-58. doi: 10.1021/jm2001629. Epub 2011 Jun 8. [21599003 ]
- General Function:
- Drug transmembrane transporter activity
- Specific Function:
- Solute transporter for tetraethylammonium (TEA), 1-methyl-4-phenylpyridinium (MPP), cimetidine, N-methylnicotinamide, metformin, creatinine, guanidine, procainamide, topotecan, estrone sulfate, acyclovir, and ganciclovir. Responsible for the secretion of cationic drugs across the brush border membranes.
- Gene Name:
- SLC47A2
- Uniprot ID:
- Q86VL8
- Molecular Weight:
- 65083.915 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| IC50 | 0.3 uM | Not Available | BindingDB 50000493 |
| IC50 | 6.9 uM | Not Available | BindingDB 50000493 |
References
- Wittwer MB, Zur AA, Khuri N, Kido Y, Kosaka A, Zhang X, Morrissey KM, Sali A, Huang Y, Giacomini KM: Discovery of potent, selective multidrug and toxin extrusion transporter 1 (MATE1, SLC47A1) inhibitors through prescription drug profiling and computational modeling. J Med Chem. 2013 Feb 14;56(3):781-95. doi: 10.1021/jm301302s. Epub 2013 Jan 22. [23241029 ]
- Kido Y, Matsson P, Giacomini KM: Profiling of a prescription drug library for potential renal drug-drug interactions mediated by the organic cation transporter 2. J Med Chem. 2011 Jul 14;54(13):4548-58. doi: 10.1021/jm2001629. Epub 2011 Jun 8. [21599003 ]
- General Function:
- Ligand-gated ion channel activity
- Specific Function:
- After binding acetylcholine, the AChR responds by an extensive change in conformation that affects all subunits and leads to opening of an ion-conducting channel across the plasma membrane permeable to sodium ions.
- Gene Name:
- CHRNA4
- Uniprot ID:
- P43681
- Molecular Weight:
- 69956.47 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| Inhibitory | 46 uM | Not Available | BindingDB 50000493 |
| IC50 | 66 uM | Not Available | BindingDB 50000493 |
References
- Papke RL, Porter Papke JK, Rose GM: Activity of alpha7-selective agonists at nicotinic and serotonin 5HT3 receptors expressed in Xenopus oocytes. Bioorg Med Chem Lett. 2004 Apr 19;14(8):1849-53. [15050614 ]
- Macor JE, Gurley D, Lanthorn T, Loch J, Mack RA, Mullen G, Tran O, Wright N, Gordon JC: The 5-HT3 antagonist tropisetron (ICS 205-930) is a potent and selective alpha7 nicotinic receptor partial agonist. Bioorg Med Chem Lett. 2001 Feb 12;11(3):319-21. [11212100 ]
- General Function:
- Secondary active organic cation transmembrane transporter activity
- Specific Function:
- Translocates a broad array of organic cations with various structures and molecular weights including the model compounds 1-methyl-4-phenylpyridinium (MPP), tetraethylammonium (TEA), N-1-methylnicotinamide (NMN), 4-(4-(dimethylamino)styryl)-N-methylpyridinium (ASP), the endogenous compounds choline, guanidine, histamine, epinephrine, adrenaline, noradrenaline and dopamine, and the drugs quinine, and metformin. The transport of organic cations is inhibited by a broad array of compounds like tetramethylammonium (TMA), cocaine, lidocaine, NMDA receptor antagonists, atropine, prazosin, cimetidine, TEA and NMN, guanidine, cimetidine, choline, procainamide, quinine, tetrabutylammonium, and tetrapentylammonium. Translocates organic cations in an electrogenic and pH-independent manner. Translocates organic cations across the plasma membrane in both directions. Transports the polyamines spermine and spermidine. Transports pramipexole across the basolateral membrane of the proximal tubular epithelial cells. The choline transport is activated by MMTS. Regulated by various intracellular signaling pathways including inhibition by protein kinase A activation, and endogenously activation by the calmodulin complex, the calmodulin-dependent kinase II and LCK tyrosine kinase.
- Gene Name:
- SLC22A1
- Uniprot ID:
- O15245
- Molecular Weight:
- 61153.345 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| IC50 | >100 uM | Not Available | BindingDB 50000493 |
| IC50 | >300 uM | Not Available | BindingDB 50000493 |
References
- Kido Y, Matsson P, Giacomini KM: Profiling of a prescription drug library for potential renal drug-drug interactions mediated by the organic cation transporter 2. J Med Chem. 2011 Jul 14;54(13):4548-58. doi: 10.1021/jm2001629. Epub 2011 Jun 8. [21599003 ]
- Wittwer MB, Zur AA, Khuri N, Kido Y, Kosaka A, Zhang X, Morrissey KM, Sali A, Huang Y, Giacomini KM: Discovery of potent, selective multidrug and toxin extrusion transporter 1 (MATE1, SLC47A1) inhibitors through prescription drug profiling and computational modeling. J Med Chem. 2013 Feb 14;56(3):781-95. doi: 10.1021/jm301302s. Epub 2013 Jan 22. [23241029 ]
- General Function:
- Quaternary ammonium group transmembrane transporter activity
- Specific Function:
- Mediates tubular uptake of organic compounds from circulation. Mediates the influx of agmatine, dopamine, noradrenaline (norepinephrine), serotonin, choline, famotidine, ranitidine, histamin, creatinine, amantadine, memantine, acriflavine, 4-[4-(dimethylamino)-styryl]-N-methylpyridinium ASP, amiloride, metformin, N-1-methylnicotinamide (NMN), tetraethylammonium (TEA), 1-methyl-4-phenylpyridinium (MPP), cimetidine, cisplatin and oxaliplatin. Cisplatin may develop a nephrotoxic action. Transport of creatinine is inhibited by fluoroquinolones such as DX-619 and LVFX. This transporter is a major determinant of the anticancer activity of oxaliplatin and may contribute to antitumor specificity.
- Gene Name:
- SLC22A2
- Uniprot ID:
- O15244
- Molecular Weight:
- 62579.99 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| IC50 | 0.89 uM | Not Available | BindingDB 50000493 |
| IC50 | 1.7 uM | Not Available | BindingDB 50000493 |
References
- Kido Y, Matsson P, Giacomini KM: Profiling of a prescription drug library for potential renal drug-drug interactions mediated by the organic cation transporter 2. J Med Chem. 2011 Jul 14;54(13):4548-58. doi: 10.1021/jm2001629. Epub 2011 Jun 8. [21599003 ]
- Wittwer MB, Zur AA, Khuri N, Kido Y, Kosaka A, Zhang X, Morrissey KM, Sali A, Huang Y, Giacomini KM: Discovery of potent, selective multidrug and toxin extrusion transporter 1 (MATE1, SLC47A1) inhibitors through prescription drug profiling and computational modeling. J Med Chem. 2013 Feb 14;56(3):781-95. doi: 10.1021/jm301302s. Epub 2013 Jan 22. [23241029 ]
- General Function:
- Serotonin receptor activity
- Specific Function:
- G-protein coupled receptor for 5-hydroxytryptamine (serotonin). Also functions as a receptor for various drugs and psychoactive substances. Ligand binding causes a conformation change that triggers signaling via guanine nucleotide-binding proteins (G proteins) and modulates the activity of down-stream effectors, such as adenylate cyclase. Beta-arrestin family members inhibit signaling via G proteins and mediate activation of alternative signaling pathways. Signaling inhibits adenylate cyclase activity and activates a phosphatidylinositol-calcium second messenger system that regulates the release of Ca(2+) ions from intracellular stores. Plays a role in the regulation of 5-hydroxytryptamine release and in the regulation of dopamine and 5-hydroxytryptamine metabolism. Plays a role in the regulation of dopamine and 5-hydroxytryptamine levels in the brain, and thereby affects neural activity, mood and behavior. Plays a role in the response to anxiogenic stimuli.
- Gene Name:
- HTR1A
- Uniprot ID:
- P08908
- Molecular Weight:
- 46106.335 Da
References
- van Wijngaarden I, Tulp MT, Soudijn W: The concept of selectivity in 5-HT receptor research. Eur J Pharmacol. 1990 Jun 12;188(6):301-12. [2164935 ]
- General Function:
- Serotonin receptor activity
- Specific Function:
- G-protein coupled receptor for 5-hydroxytryptamine (serotonin). Also functions as a receptor for ergot alkaloid derivatives, various anxiolytic and antidepressant drugs and other psychoactive substances, such as lysergic acid diethylamide (LSD). Ligand binding causes a conformation change that triggers signaling via guanine nucleotide-binding proteins (G proteins) and modulates the activity of down-stream effectors, such as adenylate cyclase. Signaling inhibits adenylate cyclase activity. Arrestin family members inhibit signaling via G proteins and mediate activation of alternative signaling pathways. Regulates the release of 5-hydroxytryptamine, dopamine and acetylcholine in the brain, and thereby affects neural activity, nociceptive processing, pain perception, mood and behavior. Besides, plays a role in vasoconstriction of cerebral arteries.
- Gene Name:
- HTR1B
- Uniprot ID:
- P28222
- Molecular Weight:
- 43567.535 Da
References
- van Wijngaarden I, Tulp MT, Soudijn W: The concept of selectivity in 5-HT receptor research. Eur J Pharmacol. 1990 Jun 12;188(6):301-12. [2164935 ]
- General Function:
- Serotonin receptor activity
- Specific Function:
- This is one of the several different receptors for 5-hydroxytryptamine (serotonin), a biogenic hormone that functions as a neurotransmitter, a hormone, and a mitogen. The activity of this receptor is mediated by G proteins that stimulate adenylate cyclase.
- Gene Name:
- HTR4
- Uniprot ID:
- Q13639
- Molecular Weight:
- 43760.975 Da
References
- van Wijngaarden I, Tulp MT, Soudijn W: The concept of selectivity in 5-HT receptor research. Eur J Pharmacol. 1990 Jun 12;188(6):301-12. [2164935 ]
- General Function:
- Serotonin receptor activity
- Specific Function:
- This is one of the several different receptors for 5-hydroxytryptamine (serotonin), a biogenic hormone that functions as a neurotransmitter, a hormone, and a mitogen. The activity of this receptor is mediated by G proteins that stimulate adenylate cyclase. It has a high affinity for tricyclic psychotropic drugs (By similarity). Controls pyramidal neurons migration during corticogenesis, through the regulation of CDK5 activity (By similarity). Is an activator of TOR signaling (PubMed:23027611).
- Gene Name:
- HTR6
- Uniprot ID:
- P50406
- Molecular Weight:
- 46953.625 Da
References
- Chen X, Ji ZL, Chen YZ: TTD: Therapeutic Target Database. Nucleic Acids Res. 2002 Jan 1;30(1):412-5. [11752352 ]
- General Function:
- Vitamin d3 25-hydroxylase activity
- Specific Function:
- Cytochromes P450 are a group of heme-thiolate monooxygenases. In liver microsomes, this enzyme is involved in an NADPH-dependent electron transport pathway. It performs a variety of oxidation reactions (e.g. caffeine 8-oxidation, omeprazole sulphoxidation, midazolam 1'-hydroxylation and midazolam 4-hydroxylation) of structurally unrelated compounds, including steroids, fatty acids, and xenobiotics. Acts as a 1,8-cineole 2-exo-monooxygenase. The enzyme also hydroxylates etoposide (PubMed:11159812). Catalyzes 4-beta-hydroxylation of cholesterol. May catalyze 25-hydroxylation of cholesterol in vitro (PubMed:21576599).
- Gene Name:
- CYP3A4
- Uniprot ID:
- P08684
- Molecular Weight:
- 57342.67 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| IC50 | 11 uM | Not Available | BindingDB 50000493 |
References
- Yang Z, Fairfax DJ, Maeng JH, Masih L, Usyatinsky A, Hassler C, Isaacson S, Fitzpatrick K, DeOrazio RJ, Chen J, Harding JP, Isherwood M, Dobritsa S, Christensen KL, Wierschke JD, Bliss BI, Peterson LH, Beer CM, Cioffi C, Lynch M, Rennells WM, Richards JJ, Rust T, Khmelnitsky YL, Cohen ML, Manning DD: Discovery of 2-substituted benzoxazole carboxamides as 5-HT3 receptor antagonists. Bioorg Med Chem Lett. 2010 Nov 15;20(22):6538-41. doi: 10.1016/j.bmcl.2010.09.038. Epub 2010 Sep 16. [20889341 ]
- General Function:
- Voltage-gated calcium channel activity
- Specific Function:
- Receptor for endogenous opioids such as beta-endorphin and endomorphin. Receptor for natural and synthetic opioids including morphine, heroin, DAMGO, fentanyl, etorphine, buprenorphin and methadone. Agonist binding to the receptor induces coupling to an inactive GDP-bound heterotrimeric G-protein complex and subsequent exchange of GDP for GTP in the G-protein alpha subunit leading to dissociation of the G-protein complex with the free GTP-bound G-protein alpha and the G-protein beta-gamma dimer activating downstream cellular effectors. The agonist- and cell type-specific activity is predominantly coupled to pertussis toxin-sensitive G(i) and G(o) G alpha proteins, GNAI1, GNAI2, GNAI3 and GNAO1 isoforms Alpha-1 and Alpha-2, and to a lesser extend to pertussis toxin-insensitive G alpha proteins GNAZ and GNA15. They mediate an array of downstream cellular responses, including inhibition of adenylate cyclase activity and both N-type and L-type calcium channels, activation of inward rectifying potassium channels, mitogen-activated protein kinase (MAPK), phospholipase C (PLC), phosphoinositide/protein kinase (PKC), phosphoinositide 3-kinase (PI3K) and regulation of NF-kappa-B. Also couples to adenylate cyclase stimulatory G alpha proteins. The selective temporal coupling to G-proteins and subsequent signaling can be regulated by RGSZ proteins, such as RGS9, RGS17 and RGS4. Phosphorylation by members of the GPRK subfamily of Ser/Thr protein kinases and association with beta-arrestins is involved in short-term receptor desensitization. Beta-arrestins associate with the GPRK-phosphorylated receptor and uncouple it from the G-protein thus terminating signal transduction. The phosphorylated receptor is internalized through endocytosis via clathrin-coated pits which involves beta-arrestins. The activation of the ERK pathway occurs either in a G-protein-dependent or a beta-arrestin-dependent manner and is regulated by agonist-specific receptor phosphorylation. Acts as a class A G-protein coupled receptor (GPCR) which dissociates from beta-arrestin at or near the plasma membrane and undergoes rapid recycling. Receptor down-regulation pathways are varying with the agonist and occur dependent or independent of G-protein coupling. Endogenous ligands induce rapid desensitization, endocytosis and recycling whereas morphine induces only low desensitization and endocytosis. Heterooligomerization with other GPCRs can modulate agonist binding, signaling and trafficking properties. Involved in neurogenesis. Isoform 12 couples to GNAS and is proposed to be involved in excitatory effects. Isoform 16 and isoform 17 do not bind agonists but may act through oligomerization with binding-competent OPRM1 isoforms and reduce their ligand binding activity.
- Gene Name:
- OPRM1
- Uniprot ID:
- P35372
- Molecular Weight:
- 44778.855 Da
References
- van Wijngaarden I, Tulp MT, Soudijn W: The concept of selectivity in 5-HT receptor research. Eur J Pharmacol. 1990 Jun 12;188(6):301-12. [2164935 ]
- General Function:
- Toxin transporter activity
- Specific Function:
- Mediates potential-dependent transport of a variety of organic cations. May play a significant role in the disposition of cationic neurotoxins and neurotransmitters in the brain.
- Gene Name:
- SLC22A3
- Uniprot ID:
- O75751
- Molecular Weight:
- 61279.485 Da
Binding/Activity Constants
| Type | Value | Assay Type | Assay Source |
|---|---|---|---|
| IC50 | 17.4 uM | Not Available | BindingDB 50000493 |
References
- Wittwer MB, Zur AA, Khuri N, Kido Y, Kosaka A, Zhang X, Morrissey KM, Sali A, Huang Y, Giacomini KM: Discovery of potent, selective multidrug and toxin extrusion transporter 1 (MATE1, SLC47A1) inhibitors through prescription drug profiling and computational modeling. J Med Chem. 2013 Feb 14;56(3):781-95. doi: 10.1021/jm301302s. Epub 2013 Jan 22. [23241029 ]