| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2009-07-21 20:28:02 UTC |
|---|
| Update Date | 2014-12-24 20:25:53 UTC |
|---|
| Accession Number | T3D2941 |
|---|
| Identification |
|---|
| Common Name | Hydrocodone |
|---|
| Class | Small Molecule |
|---|
| Description | Hydrocodone is only found in individuals that have used or taken this drug. It is a narcotic analgesic related to codeine, but more potent and more addicting by weight. It is used also as cough suppressant. [PubChem]Hydrocodone acts as a weak agonist at OP1, OP2, and OP3 opiate receptors within the central nervous system (CNS). Hydrocodone primarily affects OP3 receptors, which are coupled with G-protein receptors and function as modulators, both positive and negative, of synaptic transmission via G-proteins that activate effector proteins. Binding of the opiate stimulates the exchange of GTP for GDP on the G-protein complex. As the effector system is adenylate cyclase and cAMP located at the inner surface of the plasma membrane, opioids decrease intracellular cAMP by inhibiting adenylate cyclase. Subsequently, the release of nociceptive neurotransmitters such as substance P, GABA, dopamine, acetylcholine, and noradrenaline is inhibited. Opioids such as hydrocodone also inhibit the release of vasopressin, somatostatin, insulin, and glucagon. Opioids close N-type voltage-operated calcium channels (OP2-receptor agonist) and open calcium-dependent inwardly rectifying potassium channels (OP3 and OP1 receptor agonist). This results in hyperpolarization and reduced neuronal excitability. |
|---|
| Compound Type | - Amine
- Analgesic
- Analgesic, Opioid
- Antitussive Agent
- Drug
- Ether
- Metabolite
- Narcotic
- Organic Compound
- Synthetic Compound
|
|---|
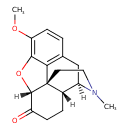
| Chemical Structure | |
|---|
| Synonyms | | Synonym | | (-)-Dihydrocodeinone | | 4,5-alpha-Epoxy-3-methoxy-17-methylmorphinan-6-one | | Dihydrocodeinone | | Hidrocodona | | Hydrocodon | | Hydrocodonum | | Hydrocone | | Hydroconum | | Idrocodone | | Reprexain | | Zohydro |
|
|---|
| Chemical Formula | C18H21NO3 |
|---|
| Average Molecular Mass | 299.364 g/mol |
|---|
| Monoisotopic Mass | 299.152 g/mol |
|---|
| CAS Registry Number | 125-29-1 |
|---|
| IUPAC Name | (1S,5R,13R,17R)-10-methoxy-4-methyl-12-oxa-4-azapentacyclo[9.6.1.0¹,¹³.0⁵,¹⁷.0⁷,¹⁸]octadeca-7(18),8,10-trien-14-one |
|---|
| Traditional Name | hydrocodone |
|---|
| SMILES | [H][C@@]12OC3=C(OC)C=CC4=C3[C@@]11CCN(C)[C@]([H])(C4)[C@]1([H])CCC2=O |
|---|
| InChI Identifier | InChI=1S/C18H21NO3/c1-19-8-7-18-11-4-5-13(20)17(18)22-16-14(21-2)6-3-10(15(16)18)9-12(11)19/h3,6,11-12,17H,4-5,7-9H2,1-2H3/t11-,12+,17-,18-/m0/s1 |
|---|
| InChI Key | InChIKey=LLPOLZWFYMWNKH-CMKMFDCUSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as morphinans. These are polycyclic compounds with a four-ring skeleton with three condensed six-member rings forming a partially hydrogenated phenanthrene moiety, one of which is aromatic while the two others are alicyclic. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Alkaloids and derivatives |
|---|
| Class | Morphinans |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Morphinans |
|---|
| Alternative Parents | |
|---|
| Substituents | - Morphinan
- Phenanthrene
- Isoquinolone
- Tetralin
- Coumaran
- Anisole
- Alkyl aryl ether
- Aralkylamine
- Piperidine
- Benzenoid
- Ketone
- Tertiary amine
- Tertiary aliphatic amine
- Oxacycle
- Ether
- Azacycle
- Organoheterocyclic compound
- Amine
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Organic oxide
- Organic nitrogen compound
- Organopnictogen compound
- Carbonyl group
- Organic oxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Exogenous |
|---|
| Cellular Locations | - Cytoplasm
- Extracellular
- Membrane
|
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | |
|---|
| Biological Roles | |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Appearance | White powder. |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | 198°C | | Boiling Point | Not Available | | Solubility | Insoluble | | LogP | 1.2 |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | Deposition Date | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0a4i-2090000000-117774746c444c4e0488 | 2017-09-01 | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , positive | splash10-0udi-0009000000-f21858df54194757e2d9 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , positive | splash10-0udi-0009000000-9b129b5d29df52345272 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , positive | splash10-0udi-0649000000-82623b23b75f0a0b232d | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , positive | splash10-0002-0920000000-b67ff5a32d0ddd40c4c7 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , positive | splash10-006t-0900000000-0277ba7435a4081ab333 | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , positive | splash10-0g6v-0900000000-b335c52fad1274cd28cb | 2017-09-14 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 35V, Positive | splash10-0udi-0539000000-60e3ee4cd365c32565d2 | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 90V, Positive | splash10-0g6v-0900000000-a3ca9b5bf55ce8c114d5 | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 45V, Positive | splash10-0udi-0539000000-a9095e27f0dee030ee9a | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 30V, Positive | splash10-0udi-0009000000-dfe701f042e4ae48fa1d | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 60V, Positive | splash10-0002-0921000000-93f2238f422f7dbee100 | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 75V, Positive | splash10-006t-0900000000-85ee173792972a321cc1 | 2021-09-20 | View Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 15V, Positive | splash10-0udi-0009000000-c527bc2c918e346dafb8 | 2021-09-20 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0029000000-1c3fa3b4b1357be62616 | 2016-06-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-1098000000-f000ea847c9e68994f23 | 2016-06-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-8090000000-dd4e0e79adfda3b828cc | 2016-06-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0090000000-d9ab1faf7327dd439b2c | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-0090000000-2b3b1b2934a4489426fc | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-003r-1190000000-666f47c119770568eaf1 | 2016-08-03 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0009000000-1d8c7ea64ca3af7aef5e | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-0009000000-8b328c7a2ca4dc1b0c72 | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0f6x-2091000000-71570a929a535e2fa4d2 | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0090000000-cf6f68fb1a2e8ad8e416 | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-0090000000-cf6f68fb1a2e8ad8e416 | 2021-10-11 | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000t-0090000000-0f770b43a2251fd7dba1 | 2021-10-11 | View Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-0005-6970000000-3ba3e105098639cd6091 | 2014-09-20 | View Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Oral. Well absorbed from the gastrointestinal tract. |
|---|
| Mechanism of Toxicity | Hydrocodone acts as a weak agonist at OP1, OP2, and OP3 opiate receptors within the central nervous system (CNS). Hydrocodone primarily affects OP3 receptors, which are coupled with G-protein receptors and function as modulators, both positive and negative, of synaptic transmission via G-proteins that activate effector proteins. Binding of the opiate stimulates the exchange of GTP for GDP on the G-protein complex. As the effector system is adenylate cyclase and cAMP located at the inner surface of the plasma membrane, opioids decrease intracellular cAMP by inhibiting adenylate cyclase. Subsequently, the release of nociceptive neurotransmitters such as substance P, GABA, dopamine, acetylcholine, and noradrenaline is inhibited. Opioids such as hydrocodone also inhibit the release of vasopressin, somatostatin, insulin, and glucagon. Opioids close N-type voltage-operated calcium channels (OP2-receptor agonist) and open calcium-dependent inwardly rectifying potassium channels (OP3 and OP1 receptor agonist). This results in hyperpolarization and reduced neuronal excitability. |
|---|
| Metabolism | Hepatic and also in intestinal mucosa.
Half Life: 1.25-3 hours |
|---|
| Toxicity Values | LD50: 85.7mg/kg (Parenteral-subcutaneous, Mouse) (1) |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | No indication of carcinogenicity to humans (not listed by IARC). |
|---|
| Uses/Sources | For relief of moderate to moderately severe pain. Also used for the symptomatic relief of nonproductive cough, alone or in combination with other antitussives or expectorants. |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Medical problems can include congested lungs, liver disease, tetanus, infection of the heart valves, skin abscesses, anemia and pneumonia. Death can occur from overdose. |
|---|
| Symptoms | Symptoms of overdose include respiratory depression (a decrease in respiratory rate and/or tidal volume, Cheyne-Stokes respiration, cyanosis), extreme somnolence progressing to stupor or coma, skeletal muscle flaccidity, dizziness, ringing in the ears, confusion, blurred vision, eye problems, cold and clammy skin, and sometimes bradycardia and hypotension. In severe overdose, apnea, circulatory collapse, cardiac arrest and death may occur. |
|---|
| Treatment | Immediate treatment includes support of cardiorespiratory function and measures to reduce drug absorption. Vomiting should be induced mechanically, or with syrup of ipecac, if the patient is alert (adequate pharyngeal and laryngeal reflexes). Oral activated charcoal (1 g/kg) should follow gastric emptying. The first dose should be accompanied by an appropriate cathartic. If repeated doses are used, the cathartic might be included with alternate doses as required. Hypotension is usually hypovolemic and should respond to fluids. Vasopressors and other supportive measures should be employed as indicated. A cuffed endo-tracheal tube should be inserted before gastric lavage of the unconscious patient and, when necessary, to provide assisted respiration. Meticulous attention should be given to maintaining adequate pulmonary ventilation. In severe cases of intoxication, peritoneal dialysis, or preferably hemodialysis may be considered. Naloxone, a narcotic antagonist, can reverse respiratory depression and coma associated with opioid overdose. Naloxone hydrochloride 0.4 mg to 2 mg is given parenterally. Since the duration of action of hydrocodone may exceed that of the naloxone, the patient should be kept under continuous surveillance and repeated doses of the antagonist should be administered as needed to maintain adequate respiration. (3) |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB00956 |
|---|
| HMDB ID | HMDB15091 |
|---|
| PubChem Compound ID | 5284569 |
|---|
| ChEMBL ID | CHEMBL1457 |
|---|
| ChemSpider ID | 4447623 |
|---|
| KEGG ID | C08024 |
|---|
| UniProt ID | Not Available |
|---|
| OMIM ID | |
|---|
| ChEBI ID | 5779 |
|---|
| BioCyc ID | Not Available |
|---|
| CTD ID | Not Available |
|---|
| Stitch ID | Hydrocodone |
|---|
| PDB ID | Not Available |
|---|
| ACToR ID | Not Available |
|---|
| Wikipedia Link | Hydrocodone |
|---|
| References |
|---|
| Synthesis Reference | Anne M. Hailes, Christopher E. French, Neil C. Bruce, “Morphinone reductase for the preparation of hydromorphone and hydrocodone.” U.S. Patent US5571685, issued November, 1990. |
|---|
| MSDS | Link |
|---|
| General References | - Wishart DS, Knox C, Guo AC, Cheng D, Shrivastava S, Tzur D, Gautam B, Hassanali M: DrugBank: a knowledgebase for drugs, drug actions and drug targets. Nucleic Acids Res. 2008 Jan;36(Database issue):D901-6. Epub 2007 Nov 29. [18048412 ]
- Drugs.com [Link]
- RxList: The Internet Drug Index (2009). [Link]
|
|---|
| Gene Regulation |
|---|
| Up-Regulated Genes | Not Available |
|---|
| Down-Regulated Genes | Not Available |
|---|